What Is Energy And How Does It Relate To Work?
Introduction to Energy
Energy is the ability to do work or produce heat. It exists in many different forms that can be grouped into two main categories – potential energy and kinetic energy. Potential energy describes stored energy based on an object’s position or arrangement, while kinetic energy is the energy of motion. According to the law of conservation of energy, energy cannot be created or destroyed, only transformed from one form to another.
Some common forms of energy include:
- Kinetic energy – the energy of motion. Examples are the energy of a moving bullet, the motion of atoms in heat, and the motion of electrons in electricity.
- Potential energy – stored energy based on an object’s position. A boulder perched atop a hill or water held behind a dam are examples of potential energy.
- Mechanical energy – the sum of an object’s kinetic and potential energy.
- Thermal energy – the energy that comes from the motion of atoms and molecules, often referred to as heat energy.
- Chemical energy – the energy stored in the bonds between atoms and molecules. Examples include biomass, petroleum, natural gas, and propane.
- Electrical energy – the movement of electrons. Lightning and electricity are forms of electrical energy.
- Nuclear energy – the energy stored within the nucleus of an atom. Nuclear power plants split uranium atoms to release enormous amounts of energy.
Work and Energy
Work is defined in physics as force applied over a distance. When a force acts on an object to cause displacement, work is done on the object. The amount of work (W) is calculated by multiplying the force (F) by the distance (d) the object moves parallel to the force, or W = Fd.
For example, when lifting a book from the floor to a tabletop, work is done to raise the book against the force of gravity. The force applied to lift the book times the vertical distance from the floor to the table results in the amount of work performed.
In physics, the principle of work involves transferring energy between objects. When work is done on an object, energy is transferred to that object. Energy can take many forms, such as kinetic energy, potential energy, thermal energy, etc. By doing work on an object, energy is transferred to the object, often increasing its kinetic or potential energy.
Work is directly related to energy, as the amount of work equals the amount of transferred energy. The units of work (joules) are also the units of energy. In summary, work in physics involves applying force over a distance, which results in energy being transferred between objects.
Kinetic Energy
Kinetic energy is the energy an object possesses due to its motion. The kinetic energy of an object depends on two physical quantities – the mass (m) and velocity (v) of the moving object. The kinetic energy (KE) of an object can be calculated using the following formula:
KE = 1/2 * m * v2
Where m is the mass of the object in kilograms (kg) and v is the velocity in meters per second (m/s). This shows that an increase in either mass or velocity will result in an increase in the object’s kinetic energy. Some examples of kinetic energy in everyday life include:
- A moving car
- A kicked soccer ball
- Wind
- A flowing river
In each case, the motion of the object represents kinetic energy. A car with more mass traveling at higher velocity will possess more kinetic energy than a lighter car moving slowly. Similarly, stronger winds have more kinetic energy than a light breeze. Kinetic energy is directly proportional to the object’s mass and the square of its velocity.
Potential Energy
Potential energy is the stored energy an object has due to its position or state. There are two main types of potential energy: gravitational potential energy and elastic potential energy.
Gravitational potential energy is energy that an object possesses due to its height above the ground. For example, a book sitting on a shelf has more gravitational potential energy than a book lying on the floor. As the book is raised higher, it gains more gravitational potential energy. The gravitational potential energy of an object near Earth’s surface depends on its mass and height above the ground.
Elastic potential energy is the energy stored in elastic materials that are deformed or stretched. For example, a stretched rubber band or spring has elastic potential energy. This stored elastic energy can be converted into kinetic energy if the object is released and allowed to return to its original shape. The more an elastic object is deformed, the more elastic potential energy it possesses.
Mechanical Energy
Mechanical energy is the sum of an object’s kinetic energy and potential energy. Kinetic energy is the energy associated with motion and depends on an object’s mass and velocity. Potential energy depends on an object’s position or shape and can be stored energy due to gravity, such as with a rock on top of a hill, or stored energy due to the compression or stretching of an elastic object, like a spring or rubber band.
When an object is in motion, it has kinetic energy. The faster it moves, the more kinetic energy it has. When an object is stationary but has the potential to move, it has potential energy. For example, a ball held above the ground has gravitational potential energy that can be converted into kinetic energy if it is dropped. The total mechanical energy of the ball remains constant, but can shift between potential and kinetic energy.
Mechanical energy is converted between potential and kinetic energy during the operation of many mechanical devices. For example, a roller coaster constantly switches gravitational potential energy at the top of hills into kinetic energy as it goes down slopes. The mechanical energy in the system remains the same, minus a small loss from friction converting some mechanical energy into thermal energy.
Thermal Energy
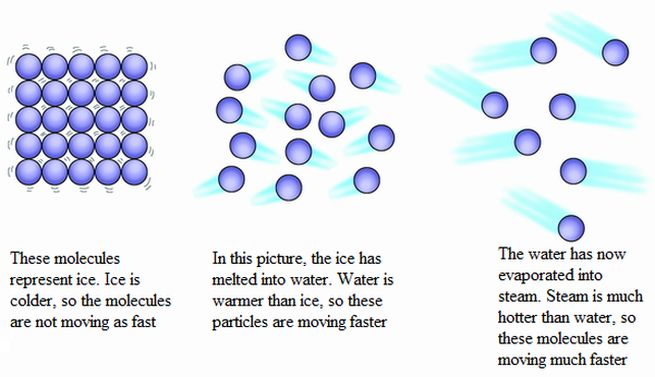
Thermal energy, also known as heat energy, is the internal energy present in matter due to the random motion and interactions of its molecules and atoms. Therefore, all matter with a temperature above absolute zero has some amount of thermal energy. The hotter an object is, the faster its molecules and atoms vibrate and move, indicating more thermal energy.
Thermal energy is a form of kinetic energy at the molecular level, as it involves the kinetic motions and vibrations of particles that make up matter. The more active the molecular motion, the higher the thermal energy. Heat is transferred between objects and systems when some kinetic energy is transferred between their particles during interactions. Thermal energy always spontaneously transfers from higher-temperature objects to lower-temperature objects until equilibrium is reached.
Common examples of thermal energy include the warmth provided by a campfire, stove, or furnace and the heat emitted by car engines and the human body. Anything that combusts chemical energy to produce a flame or heat utilizes thermal energy. Many technologies like internal combustion engines, power plants, and heating/cooling systems rely on the transfer and conversion of thermal energy.
Chemical Energy
Chemical energy is the energy stored in the bonds between atoms and molecules. It is the energy that holds these molecules together. This energy can be released when chemical bonds are broken. Some examples of chemical energy include:
- Batteries – The chemical reactions that take place in batteries involve breaking and forming of molecular bonds that release energy in the form of electricity.
- Food – Food contains high energy molecules like fats, carbohydrates and proteins. When we digest food, these molecules are broken down releasing energy our cells can use.
- Fuel – Fossil fuels like coal, oil and natural gas also contain high energy molecules. Burning these fuels breaks their molecular bonds liberating heat and light energy.
In summary, chemical energy exists in the bonds holding atoms and molecules together. This energy can be harnessed in chemical reactions when these bonds are rearranged or broken.
Electrical Energy
Electrical energy arises from the movement of electrons, usually through a conductor. Some common examples of electrical energy include batteries and wall outlets.
Batteries contain chemicals that react together to produce electrons. These electrons move through the wires and conductors inside the battery to the terminals, producing an electric current. This current can then be used to power electrical devices.
Wall outlets are connected to generators at power plants that move magnets around coils of wire to induce a current. This electricity is transmitted through power lines to homes and businesses. Plugging devices into the wall outlets connects them to this flow of electrons, powering the devices.
Other sources of electrical energy include solar panels and generators. Solar panels convert energy from sunlight directly into electricity through the photovoltaic effect. Generators use mechanical rotation to move magnets and generate electric current.
No matter how it’s produced, electrical energy is extremely useful because it can be easily transmitted through wires and converted into other useful forms of energy like heat, light, and motion.
Nuclear Energy
Nuclear energy comes from the nuclei of atoms. Nuclei contain protons and neutrons that are tightly bound together. Nuclear energy is released when the nuclei are split (fission) or fused together (fusion).
In nuclear fission, atoms like uranium or plutonium are split apart into lighter atoms, releasing energy in the process. Fission reactions are used in nuclear power plants to heat water and produce steam that spins turbines to generate electricity. The fission of uranium atoms produces a great amount of heat energy from a small amount of fuel.
Nuclear fusion joins together light nuclei to form heavier nuclei, releasing energy. The sun produces energy through fusion reactions, fusing hydrogen atoms together under intense heat and pressure to form helium. Fusion power holds the promise of providing enormous amounts of clean energy, using hydrogen extracted from water. However, controlled fusion energy for power plants remains scientifically and technologically challenging.
Nuclear power provides about 10% of the world’s electricity. While nuclear energy does not produce air pollution or carbon dioxide, concerns remain over reactor safety and radioactive waste disposal. But nuclear power offers a major low-carbon electricity source that can supplement renewable energy to provide cleaner energy worldwide.
Converting Between Energy Types
Energy can readily transform from one form into another. This allows us to convert energy into useful forms that can power our modern society. Here are some key energy conversions:
Chemical to Kinetic
Combustion engines like in cars and jets convert the chemical energy stored in fuel into kinetic energy of motion. The fuel combusts with oxygen, releasing energy that pushes pistons and turns the wheels or propellers.
Chemical to Electrical
Batteries convert stored chemical energy into electrical current that can power electronics and devices. The chemical reactions in the battery cause electrons to flow through the external circuit, providing electricity.
Nuclear to Thermal
Nuclear power plants convert nuclear energy released through fission into thermal energy that boils water into steam. The high-pressure steam then spins a turbine to generate electricity.
Mechanical to Electrical
Generators and turbines convert mechanical rotation into electrical current. The kinetic energy of the spinning coils creates changing magnetic fields that push electrons and generate electricity.
Understanding these energy conversions allows us to harness natural forms of energy like chemical, nuclear and mechanical to provide the electricity that powers modern civilization.