What Is Chemical Energy Converted To Light Energy?
Chemical energy is the potential energy stored in the bonds between atoms and molecules. It is energy derived from the oxidation (burning) of substances that contain carbon and hydrogen. Examples of substances containing chemical energy include fossil fuels like coal, oil and natural gas.
Light energy, also known as radiant energy, is a form of kinetic energy produced by electromagnetic radiation. Examples of light energy include visible light from lightbulbs, infrared radiation from hot objects, and ultraviolet radiation from the sun.
Chemical energy can be converted into light energy through exothermic chemical reactions. This occurs when the chemical bonds in a substance are broken and the atoms rearrange into different molecules, releasing energy in the process. If this released energy is in the form of electromagnetic radiation within the visible light spectrum, it will be observed as light.
Common examples of chemical to light energy conversion include fire and combustion, bioluminescence in living organisms, and chemiluminescence in chemical solutions. Each of these processes relies on chemical reactions that produce electrons in excited states that emit light when they return to ground state.
Forms of Chemical Energy
There are two main forms of chemical energy: potential and kinetic. Potential chemical energy is the energy stored within the bonds of molecules. Adenosine triphosphate (ATP) and glucose are examples of molecules with high potential chemical energy due to their chemical bonds. The breakdown of these bonds releases energy that can perform cellular work. Kinetic chemical energy is the energy of molecules in motion. The movement and collisions of molecules translates directly into kinetic energy. Higher temperatures correspond to faster molecular motion and higher kinetic energy. Both potential and kinetic chemical energies are essential sources of energy in biological and chemical systems.
Light Energy
Light energy is a form of electromagnetic radiation that is visible to the human eye. It consists of photons that travel in waves of different wavelengths. The wavelength of light determines the color that is perceived. Light wavelengths range from 380 to 700 nanometers, corresponding to the colors of the rainbow from violet to red.
The spectrum of visible light that humans can see only makes up a small portion of the full electromagnetic spectrum, which extends from radio waves to gamma rays. Other parts of the electromagnetic spectrum, like ultraviolet and infrared light, have wavelengths too short or long to be detected by the human eye.
Light energy is typically measured in discrete units called photons. The energy of a photon depends on its frequency or wavelength. Higher frequency photons have more energy. The number and energy of photons determine the brightness and color of the light. For example, blue light has a higher frequency and more energetic photons than red light.
Chemical to Light Energy Conversion
Chemical energy can be converted into light energy through exothermic chemical reactions. Exothermic reactions involve the breaking of molecular bonds, which releases energy. Some of this released energy can be emitted in the form of photons of light.
A common example of chemical to light energy conversion is combustion or oxidation reactions like fire. In fire, the oxidation of a fuel source leads to an excited electron state that relaxes by emitting photons of light energy, which we see as flames. The color of the flame depends on the emission spectrum of the atoms and molecules in the fuel source.
Bioluminescence is another example of chemicals being converted to light energy. It involves light emission from living organisms like fireflies, anglerfish, and glowworms. Bioluminescence relies on chemical reactions between luciferin and the enzyme luciferase in the presence of oxygen, which results in an excited electron state that releases photons as it returns to ground state.
Chemiluminescence is where light is produced from a chemical reaction without combustion or the presence of a living organism. Examples include glow sticks, in which hydrogen peroxide reacts with fluorescent dyes to emit light. The energy from the chemical reaction excites the dye molecules to a higher energy level, which then release photons as they return to their ground state.
Fire and Combustion
Fire is one of the most common examples of converting chemical energy to light energy. It involves the combustion or burning of a fuel source like wood, coal, natural gas or other hydrocarbons. This process is an exothermic oxidation reaction, meaning it releases energy in the form of heat and light.
Specifically, the chemical bonds in the fuel source are broken down through a reaction with oxygen. This releases energy that powers the visible light and infrared radiation we see as flames and feel as heat. The combustion of wood, for example, breaks down cellulose and lignin through an exothermic reaction. The energy released enables the remaining compounds to reach an excited state where they emit light photons.
The color and intensity of the flame depends on the fuel source and temperature. Hotter fires produce more blue and white light while cooler fires appear more red and orange. The brightness depends on the amount of energy released. Overall, fire provides a simple yet powerful example of efficiently converting the stored chemical energy in fuels into visible light through exothermic oxidation reactions.
Bioluminescence
Bioluminescence refers to light production by living organisms. It results from enzyme-catalyzed chemiluminescent reactions, where chemical energy is converted into light energy. The light-emitting enzymes are called luciferases and the light-emitting substrates are called luciferins. Bioluminescence is most commonly found in marine organisms that live in the aphotic zone where sunlight does not penetrate.
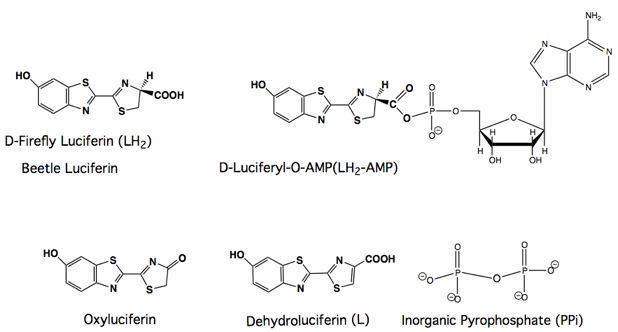
Some examples of bioluminescent organisms include:
- Fireflies – Fireflies produce bioluminescent light in their abdomen to attract mates.
- Glowworms – Glowworms (which are not actually worms) generate light through bioluminescent reactions to attract prey.
- Deep sea creatures – Many deep sea fish, jellies, squid, and shrimp utilize bioluminescence for activities like hunting, defense, and mating in the dark ocean depths.
The bioluminescent reactions in these organisms are highly efficient at producing light energy, making them a subject of fascination and scientific study.
Chemiluminescence
Chemiluminescence refers to the production of light from a chemical reaction, without heat. Unlike bioluminescence, which involves living organisms, chemiluminescence is simply a chemical process where the energy released enables certain molecules to reach an electronically excited state that emits light as it returns to the ground state.
A classic example of chemiluminescence is the reaction between luminol and blood. The iron in hemoglobin catalyzes the oxidation of luminol, causing it to emit a blue glow that can detect trace amounts of blood at crime scenes. Another common example involves combining hydrogen peroxide with horseradish extract, which contains the enzyme horseradish peroxidase. This enzyme catalyzes the oxidation of luminol or luciferin in the presence of peroxide, generating light.
Other molecules used in chemiluminescence include acridinium esters, oxalates, and acridan-based compounds. The intensity and color of light emitted depends on the chemical structure of the molecules involved. Applications of chemiluminescence include medical diagnostics, analytical chemistry, and glow sticks or light sticks which provide illumination. It offers a simple and effective way to generate light through chemical reactions.
Applications
There are several notable applications of chemical to light energy conversion:
Chemiluminescence is commonly used in glow sticks to provide portable illumination. It involves mixing chemicals in a tube to produce light emission. Chemiluminescence is also used in chemical analysis for detecting compounds, visualizing processes, and analyzing blood samples.
Bioluminescence has become an invaluable tool for studying cellular and molecular processes. Bioluminescent organisms produce light through natural enzymatic reactions. Researchers can use bioluminescent proteins as biosensors to monitor biological activities. Bioluminescence imaging is also used to track tumors, infectious diseases, and gene expression in living organisms.
Efficiency Considerations
When chemical energy is converted to light energy, the process cannot achieve 100% efficiency. Some amount of the input chemical energy will inevitably be lost or “wasted” as heat during the conversion. The efficiency of a particular chemical to light conversion depends on the kinetics and thermodynamics of the specific reaction.
Some chemical reactions that produce light occur rapidly, while others are slower. Fast reactions tend to be more efficient at channeling the energy into light, rather than losing it as heat. The kinetics of the reaction depend on parameters like temperature, pressure, and the molecular structures of the reactants.
Additionally, the theoretical maximum efficiency of any chemical to light conversion is limited by thermodynamic considerations. The amount of chemical energy available and the energy required to produce a photon of light set fundamental upper bounds on the achievable efficiency. Due to these thermodynamic limits, no chemical to light reaction can ever achieve 100% efficiency in practice.
In summary, a variety of factors influence the efficiency of converting chemical energy to light. While total efficiency is always less than 100%, careful choice of chemicals and optimization of reaction conditions can maximize the amount of chemical energy transformed into usable light.
Conclusion
In summary, the conversion of chemical energy into light energy is a natural process that occurs in many forms. The most common example is combustion or burning, where the chemical energy stored in materials like wood or oil is released as heat and light when they undergo oxidation reactions with oxygen. Bioluminescence and chemiluminescence are other processes where organisms or chemical reactions produce light through enzymatic reactions or electron excitation.
The conversion of chemical energy into light has many important applications and implications. It is essential for natural processes like photosynthesis and vision. Humans have harnessed it for lighting, heating, cooking, and more through controlled combustion. Studying these light-producing reactions also sheds light on chemistry, biology, and physics at the molecular level. Overall, the ability to convert chemical potential energy into visible light is a fascinating phenomenon that pervades many aspects of science and nature.