What Is An Example Of Energy And Matter In Chemistry?
Energy and matter are fundamental concepts in chemistry. Energy is the capacity to do work or produce heat. Matter is anything that has mass and takes up space. The relationship between energy and matter is that they can be converted into one another. This interconversion obeys two fundamental laws of thermodynamics.
The first law states that energy can neither be created nor destroyed in a chemical process, only converted from one form to another. For example, chemical energy in gasoline can be converted into kinetic energy to move a car. The total amount of energy in the universe remains constant.
The second law states that energy tends to change from being concentrated in one place to becoming more spread out and disordered over time. This natural dispersal of energy is called entropy. Although energy is conserved overall, the amount of usable energy decreases in any closed system as entropy increases.
Chemical reactions, nuclear reactions, radioactive decay, and biological processes are all examples of energy and matter interconverting in chemistry. This interplay allows chemistry to study changes in matter and energy at the atomic and molecular levels.
Laws of Thermodynamics
The laws of thermodynamics describe the relationships between heat, work, and energy in chemical systems. The first law of thermodynamics, also known as the law of conservation of energy, states that energy can neither be created nor destroyed in a chemical reaction or physical process – it can only be transferred or transformed from one form to another. For example, in an exothermic chemical reaction, the energy released by the reaction (heat) came from the energy stored in the bonds of the reactants.
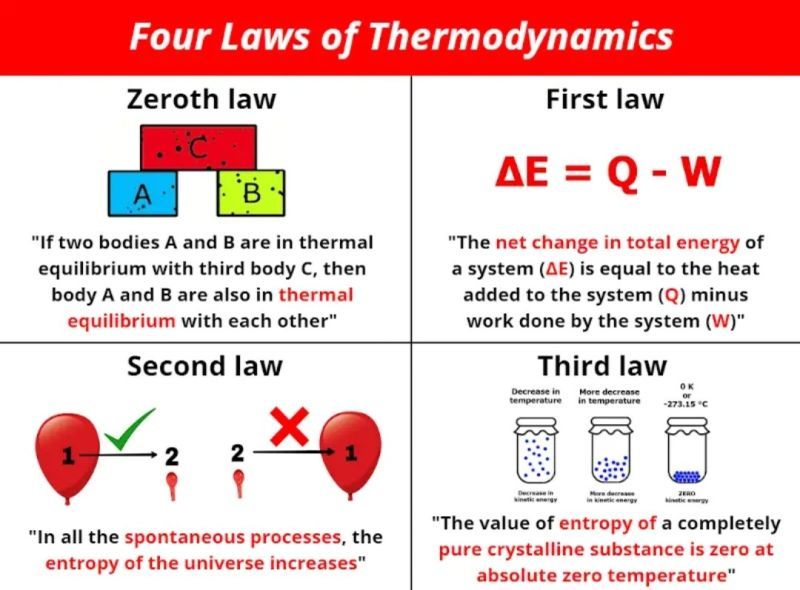
The second law of thermodynamics states that the entropy, or disorder, of an isolated system will always increase over time. Entropy is a measure of randomness or disorder at the molecular level. As chemical reactions and physical processes occur, energy disperses and becomes more disordered. For example, hot coffee left on a table eventually cools down as the heat energy dissipates into the surrounding air. According to the second law of thermodynamics, it would not be possible for this heat energy to spontaneously re-concentrate back into the coffee to make it hot again. The second law describes the one-way direction in which many chemical and physical processes proceed.
Phase Changes
One example of energy and matter interactions in chemistry is phase changes. Phase changes are the transition between different states of matter, such as solid, liquid, and gas. For a phase change to occur, a substance must either absorb or release energy.
A common phase change is solid to liquid. When a solid substance like ice is heated, it absorbs energy from its surroundings. This energy causes the molecules in the solid to start moving and break away from their fixed positions, changing the ice into liquid water. The energy input melts the solid into a liquid state.
Another phase change is liquid to gas. When liquid water is heated to its boiling point, it absorbs enough energy for the water molecules to completely break free of each other and enter the gaseous state as water vapor. Energy is required to overcome the attractions between water molecules in order to vaporize the liquid.
The reverse processes, liquid to solid and gas to liquid, release energy. As the substances cool, they lose energy to their surroundings and the molecules settle into more ordered, lower energy states. For example, when water vapor condenses into liquid water, the energy released can be seen as the heat of condensation.
Phase changes demonstrate how energy transfers enable matter to change between different physical states. The energy flows into or out of a substance depending on whether the phase change requires energy input or output.
Chemical Reactions
Chemical reactions are processes where the atomic structure of molecules is rearranged, forming new substances as a result. This rearrangement occurs by the breaking and reforming of chemical bonds between atoms. Energy is absorbed or released during the breaking and reforming of these bonds.
For example, in the chemical reaction where hydrogen and oxygen gases combine to form water:
2H2 + O2 → 2H2O
The bonds between the hydrogen atoms in H2 and between the oxygen atoms in O2 must first be broken. This requires an input of energy to break these bonds. Then, new bonds are formed between the hydrogen and oxygen atoms to create water molecules (H2O). Energy is released when these new bonds are formed.
The total energy change in a chemical reaction depends on how the energy required to break bonds compares to the energy released when new bonds form. Exothermic reactions release more energy than is required to break bonds, resulting in an overall release of energy. Endothermic reactions require more energy to break bonds than is released when new bonds form, so they absorb a net amount of energy.
In summary, chemical reactions rearrange atoms and involve the absorption or release of energy, demonstrating that matter and energy are interconverted in chemistry.
Nuclear Reactions
Nuclear reactions involve changes in the nucleus of atoms. There are two main types of nuclear reactions: nuclear fusion and nuclear fission.
Nuclear fusion occurs when two light nuclei combine to form a heavier nucleus. An example is when two hydrogen nuclei fuse to form a helium nucleus. This fusion reaction releases a tremendous amount of energy. Nuclear fusion powers the sun and other stars.
Nuclear fission occurs when a heavy nucleus splits into two or more lighter nuclei. An example is the splitting of a uranium nucleus into a barium nucleus and a krypton nucleus. Nuclear fission also releases a large amount of energy. Nuclear fission is used in nuclear power plants to generate electricity.
In both fusion and fission reactions, a small amount of mass is converted into a massive amount of energy. This demonstrates Einstein’s famous equation E=mc2, which shows the equivalence of mass and energy. Even a tiny amount of mass loss in a nuclear reaction results in an enormous release of energy.
In summary, nuclear reactions involve changes in an atom’s nucleus and demonstrate the conversion of matter into energy as described by Einstein’s theory of relativity.
Radioactive Decay
Radioactive decay occurs when an unstable nucleus spontaneously releases energy and transforms into a more stable nucleus. This happens because proton-to-neutron ratios in some isotopes are unstable. There are several modes of radioactive decay, but common ones include alpha decay, beta decay, and gamma emission.
In alpha decay, the nucleus emits an alpha particle consisting of two protons and two neutrons. This causes the original nucleus to decrease in atomic mass by 4 units and atomic number by 2 units. For example, uranium-238 can undergo alpha decay to become thorium-234.
With beta decay, the nucleus transforms by converting a neutron into a proton, emitting an electron and an antineutrino in the process. This causes the original atomic number to increase by 1 while the mass number remains constant. An example is carbon-14 decaying into nitrogen-14 by beta particle emission.
Gamma emission occurs when a nucleus in an excited energy state releases a gamma ray photon. The gamma ray carries away energy, allowing the nucleus to drop to a lower energy level. Unlike alpha and beta decay, gamma emission does not change the identity of the nucleus or alter its atomic number and mass number. It only loses energy.
The random nature of radioactive decay leads to the concept of half-lives. This is the amount of time it takes for half the nuclei in a radioactive sample to decay. Though the exact moment is unpredictable, the time it takes for half to decay is constant, allowing half-lives to indicate the rate of decay. Longer half-lives correspond to slower decay. Understanding half-lives allows scientists to properly handle radioactive materials.
Photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy from the sun into chemical energy that can be used to fuel the organism’s activities. In plants, photosynthesis occurs primarily in the leaves, where chloroplasts containing the green pigment chlorophyll reside.
The basic equation for photosynthesis is:
6CO2 + 6H2O + Light Energy → C6H12O6 + 6O2
During photosynthesis, plants take in carbon dioxide (CO2) and water (H2O) from the environment. Using energy from sunlight, the chloroplasts in plant cells convert the carbon dioxide and water into glucose (C6H12O6) and release oxygen (O2) as a byproduct. The glucose acts as an energy source that plants use to build leaves, flowers, fruits, and seeds. The oxygen is released into the atmosphere through the plant’s stomata and can then be used by other organisms for cellular respiration.
Therefore, photosynthesis converts light energy from the sun into the chemical energy stored in the glucose molecules. The energy in glucose and other carbohydrates produced by plants is passed on when organisms eat plants or other organisms that have eaten plants. In this way, nearly all energy used by living things ultimately comes from photosynthesis.
Cellular Respiration
Cellular respiration is the process by which cells convert nutrients like glucose into energy in the form of ATP. This multi-step process takes place in the cytoplasm and mitochondria of eukaryotic cells and involves both aerobic (oxygen-requiring) and anaerobic (non-oxygen requiring) pathways.
The overall chemical reaction for aerobic cellular respiration is:
C6H12O6 (glucose) + 6 O2 → 6 CO2 + 6 H2O + Energy (ATP)
There are three main stages:
1. Glycolysis – This anaerobic process breaks glucose into two pyruvate molecules, producing 2 ATP molecules per glucose molecule.
2. Krebs Cycle – The pyruvate enters the mitochondria and goes through a cyclical series of energy-extracting reactions, producing carbon dioxide and hydrogen atoms that carry electrons. This generates 2 ATP per glucose molecule.
3. Oxidative phosphorylation – The electrons from the hydrogen atoms pass through the electron transport chain in the mitochondrial membrane, powering protons to flow against their gradient which drives ATP synthase to produce around 32 ATP molecules per glucose molecule.
The large amount of ATP generated makes cellular respiration very efficient for harvesting chemical energy from organic compounds like glucose. This ATP powers most energy-requiring cellular reactions and processes in both eukaryotic and prokaryotic organisms.
Food Webs
One of the most illustrative examples of the flow of energy and matter in ecosystems is through food webs. Food webs map out the feeding relationships between organisms within an ecosystem. They show the transfer of energy and nutrients from producers (plants) up through the various trophic levels of consumers (herbivores and carnivores).
At the base of food webs are photosynthetic organisms like plants, algae and phytoplankton. Through photosynthesis, they convert light energy from the sun into chemical energy stored within organic compounds. This energy and organic matter then passes to primary consumers that eat these producers. As herbivores eat plants, some of the energy and nutrients are passed on and are incorporated into the herbivore’s own biomass.
The passing of energy continues as primary consumers are eaten by secondary consumers. As carnivores and omnivores eat other animals, they obtain energy and nutrients from the biomass of their prey. However, at each transfer between trophic levels, some energy is lost as heat. This energy loss limits the length of food chains and shapes ecosystem structure.
Therefore, food webs demonstrate how energy and matter are passed between organisms and move through ecosystems. The productivity of natural systems depends on this flow of energy and nutrients from plants up through the various consumers.
Conclusion
We have explored multiple examples that demonstrate the crucial relationships between energy and matter in chemistry. The laws of thermodynamics show that while the total amount of energy in the universe stays constant, energy can change forms and be transferred between objects through processes like heating and cooling. Phase changes illustrate how matter can transition between solid, liquid and gas while maintaining the same chemical composition. Chemical reactions rearrange atoms to form new substances, absorbing or releasing energy in the process. Nuclear processes like radioactive decay convert matter into energy according to Einstein’s famous equation E=mc2.
Photosynthesis and cellular respiration exemplify how organisms require energy from food to sustain life, while food chains display how energy flows through ecosystems. The core theme across all these examples is that energy and matter are intrinsically linked and can be converted from one form to another. This relationship between energy and matter is fundamental not just to chemistry, but to all the natural sciences and life itself. A deep appreciation of how energy and matter interact provides insight into our universe at scales from subatomic particles to entire galaxies.