What Is An Example Of A Chemical Source Of Electricity?
A chemical source of electricity is a process that generates electrical energy via chemical reactions, rather than combustion, solar, wind, nuclear or other common methods. As the world looks to transition away from fossil fuels, alternative and renewable energy sources become increasingly important. One promising technology is the fuel cell, which converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Fuel cells represent a clean, efficient and reliable way to produce electricity from abundant fuels like hydrogen, natural gas and alcohols.
How Fuel Cells Work
Fuel cells are electrochemical devices that convert the chemical energy from a fuel into electricity through a chemical reaction. Unlike batteries, fuel cells require a continuous source of fuel and oxygen to run, and do not need recharging. The fuel is supplied to the anode, where a catalyst causes the fuel to undergo oxidation reactions that generate electrons and protons. The electrons are forced through a circuit, generating an electric current that can be utilized before it returns to the cathode. Meanwhile, the protons move through the electrolyte to the cathode. At the same time, oxygen is supplied to the cathode and, in the presence of the electrolyte, will cause reduction reactions that attract the electrons back from the circuit. This whole process generates an electrical current that can be put to practical use.
The most common type of fuel cell uses hydrogen as the fuel and oxygen as the oxidant. The chemical reaction that takes place in the cell is:
Anode reaction: 2H2 → 4H+ + 4e-
Cathode reaction: O2 + 4H+ + 4e- → 2H2O
Overall reaction: 2H2 + O2 → 2H2O
This reaction produces electricity, water and heat. The electrolyte allows the protons to travel from the anode to the cathode while keeping the gases separate. The catalysts, usually made of platinum, are needed to speed up the reactions at the electrodes.
Types of Fuel Cells
There are several different types of fuel cells that vary based on the electrolyte used and the operating temperature:
Polymer Electrolyte Membrane (PEM) Fuel Cell
PEM fuel cells use a water-based, acidic polymer membrane as the electrolyte. They operate at relatively low temperatures of around 80°C, which allows them to start up quickly. PEM fuel cells are best suited for transport applications as well as small stationary uses.
Solid Oxide Fuel Cell (SOFC)
SOFCs use a hard, ceramic compound of metal oxides as the electrolyte. They operate at very high temperatures of 500°C to 1,000°C. This high temperature operation eliminates the need for precious metal catalysts like platinum. The high temperatures allow SOFCs to use a variety of fuels like natural gas. SOFCs are being developed for both stationary and mobile applications.
Phosphoric Acid Fuel Cell (PAFC)
PAFCs utilize liquid phosphoric acid as the electrolyte. They operate at moderately high temperatures of 150°C to 200°C. This temperature range allows them to have higher efficiencies than PEM fuel cells. PAFCs are typically used for stationary power generation.
Molten Carbonate Fuel Cell (MCFC)
MCFCs employ a molten carbonate salt mixture suspended in a ceramic lithium aluminum oxide matrix as the electrolyte. They operate at extremely high temperatures of 650°C. The high temperatures enable the use of abundant and affordable catalysts. MCFCs are ideally suited for natural gas and coal-based power plants.
In summary, fuel cell types differ based on operating temperature and electrolyte used. Lower temperature PEM cells are well-suited for transport applications. Moderate temperature PAFCs are primarily for stationary uses. High temperature SOFCs and MCFCs can utilize a wider range of fuels and are suitable for both stationary and some mobile applications.
Applications of Fuel Cells
Fuel cells have a wide range of applications due to their clean and efficient production of electricity. Some of the main uses are:
Vehicles
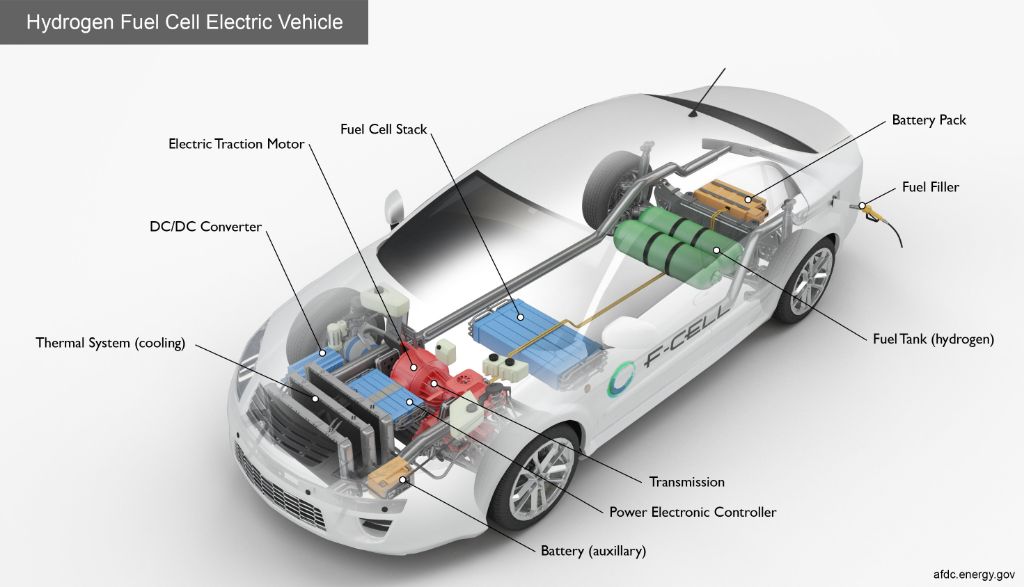
Fuel cell vehicles like cars, buses, forklifts and submarines utilize hydrogen fuel cells as their power source. Fuel cell vehicles have greater efficiency and reduce greenhouse gas emissions compared to internal combustion engines. They can offer driving ranges over 300 miles between refueling.
Backup Power
Fuel cells can provide reliable backup power to businesses, hospitals, and even households. Stationary fuel cells are used as on-site electricity generation to provide emergency power, UPS systems, and primary energy for remote locations.
Portable Electronics
Small fuel cells can power portable devices like smartphones, laptops, cameras and drones. The fuel cells extend battery life by providing power anywhere, anytime. This gives great benefit over batteries which need recharging.
Overall, fuel cells present many advantages over combustion generators and batteries. They have higher efficiency with lower emissions, can quickly respond to demand fluctuations, and avoid the need for energy storage. These characteristics make fuel cells ideal for vehicles, backup power and portable electronics.
Advantages of Fuel Cells
Fuel cells offer several key advantages that make them an appealing source of electricity:
Clean, efficient source of electricity – Unlike combustion-based power plants, fuel cells produce electricity through a chemical reaction, not combustion. This makes them very clean – their only byproducts are water and heat. Fuel cells can achieve efficiencies of up to 60%, much higher than traditional power plants.
Reliable energy production – Fuel cells operate as long as fuel is supplied, providing uninterrupted power. Their lack of moving parts also results in reliable, consistent energy production.
Lower maintenance costs – The absence of moving parts also equates to lower maintenance costs compared to other technologies.
Modular and scalable – Fuel cells are modular, meaning units can be combined to form stacks that can be sized to serve different applications – from powering small devices to providing electricity for entire buildings or communities. This inherent scalability makes fuel cells highly versatile.
Disadvantages of Fuel Cells
While fuel cells offer many benefits, they also come with some drawbacks that need to be considered:
Expensive materials – Fuel cells rely on precious metals like platinum as catalysts. These rare materials drive up the cost of production. Making fuel cells economical on a large scale requires reducing the amount of platinum used.
Slow startup times – It takes time for fuel cells to reach operating temperature. This lag can be problematic for applications that require rapid startup, like cars. Improved system design is needed to address this.
Production of waste heat – Like all energy conversions, fuel cells generate heat. Managing this thermal output presents engineering challenges, especially for small-scale applications.
Hydrogen storage issues – Storing enough hydrogen onboard a vehicle to provide adequate driving range remains an obstacle. Hydrogen has low energy density per volume, so pressurized tanks are needed.
Comparison to Batteries
Fuel cells differ from batteries in some key ways. Batteries are energy storage devices, as they store electrical energy chemically and convert it back to electricity on demand. In contrast, fuel cells are energy conversion devices that utilize a continuous source of fuel and oxidant to generate electricity.
Batteries have a lower energy density than most fuels, meaning a smaller amount of energy is stored per unit volume or mass. For example, lithium-ion batteries may store 0.36–0.875 MJ/kg, while hydrogen fuel stores about 120 MJ/kg, over 100 times more. This allows fuel cell systems to potentially offer longer runtimes between refueling.
However, batteries benefit from being rechargeable, while fuel cells require regular refueling. Most fuel cells also require expensive catalysts and other materials like platinum, whereas batteries use more common materials like lithium and cobalt. On the other hand, batteries degrade over time after hundreds of charge/discharge cycles, while fuel cells can theoretically operate indefinitely with proper maintenance.
In summary, batteries and fuel cells both have advantages and disadvantages in converting chemical energy to electricity. Batteries are self-contained and rechargeable, while fuel cells can offer higher energy densities but require refueling. The optimal technology depends on the specific application and its power needs.
Latest Developments
Fuel cell technology has come a long way in recent decades, but ongoing research aims to further improve the cost, efficiency, and durability of fuel cells.
Several new electrolyte materials have been developed that allow fuel cells to operate at lower temperatures without external humidification. This reduces costs and extends the operating lifetime of fuel cells.
New catalysts using platinum alloys and core-shell structures can improve efficiency and reduce the amount of costly platinum required. Advanced manufacturing techniques like nanoimprinting allow production of highly uniform and efficient electrodes.
Automakers like Toyota and Honda have commercialized fuel cell vehicles in limited production runs. Hyundai recently unveiled the Nexo, a fuel cell SUV with 370 miles of range. As production scales up, costs are projected to come down.
Stationary fuel cells for backup power, microgrids, and primary power are also being deployed. Companies like Bloom Energy, Doosan Fuel Cell, and FuelCell Energy manufacture commercial-scale fuel cell systems with electrical efficiencies over 60%.
With continued research and growing adoption across transport, power, and portable applications, fuel cells are poised to play an expanding role in sustainable energy solutions.
Future Outlook
The future looks bright for fuel cells. As production scales up, costs are projected to fall, allowing wider adoption across transportation, portable, and stationary applications. Some experts estimate that fuel cell electric vehicles could reach cost parity with internal combustion engines in the next 5-10 years as manufacturing techniques improve. This could accelerate the transition away from fossil fuels in the automotive sector.
If costs keep declining, fuel cells could also play a major role in reinventing the power grid. By generating electricity where it’s consumed, fuel cells can reduce the load on centralized power plants and transmission infrastructure. Miniaturized units could provide backup power or off-grid electricity solutions. Fuel cell power plants could even replace coal and natural gas stations at grid scale.
However, some challenges remain. Hydrogen production and distribution infrastructure needs major investment and expansion for the hydrogen economy vision to be fully realized. Fuel cell durability and reliability will need to improve as more real-world data is collected over long-term operation. And further research into cheaper materials and manufacturing methods will help drive costs down. But the technology holds significant promise for cleaner, more resilient energy across applications.
Conclusion
In summary, fuel cells are an important chemical source of electricity that convert the chemical energy from a fuel into electricity through a chemical reaction. The most common types of fuel cells are polymer electrolyte membrane (PEMFC), direct methanol (DMFC), phosphoric acid (PAFC), molten carbonate (MCFC), and solid oxide (SOFC). Fuel cells are used for a variety of applications including transportation, portable electronics, and stationary power generation.
Compared to traditional batteries, fuel cells can offer higher efficiencies, lower emissions, quiet operation, and reduced reliance on fossil fuels. While batteries store energy, fuel cells can continuously generate electricity as long as fuel and oxidant are supplied. This makes them well suited for renewable energy storage and electric vehicle applications.
Ongoing research and development aims to further improve fuel cell performance and drive down costs. With continued progress, fuel cells have the potential to play an instrumental role in building a clean and sustainable energy future.