What Is An Acceptable Definition Of Heat?
Heat is a form of energy transfer between objects or systems that are at different temperatures. It refers to the energy that is moving from a hotter body to a colder one when the two come into contact. Heat always flows spontaneously from higher temperature to lower temperature bodies.
On a microscopic scale, heat arises from the random motions of atoms and molecules. The hotter a material is, the faster its atoms and molecules vibrate and move. As the fast-moving particles in the hotter body collide with the slower-moving particles in the colder body, energy is transferred from the hotter body to the colder one until both reach the same temperature.
Therefore, heat is a manifestation of the thermal energy possessed by particles in a substance. It flows between objects to balance their energy and temperature. Key concepts related to heat include thermal energy, temperature, calorimetry, thermodynamics, heat transfer mechanisms, and more.
Thermal Energy
Heat can be defined as thermal energy that transfers from one object or system to another due to a temperature difference. When two objects or systems come in contact with each other, energy will flow from the object/system at a higher temperature to the object/system at a lower temperature until they reach thermal equilibrium. This energy transfer between the objects, caused by the temperature difference, is referred to as heat.
Heat flows spontaneously from higher temperature to lower temperature objects or systems. The higher kinetic energy of molecules in the higher temperature object is transferred to the lower kinetic energy molecules of the cooler object when the objects make contact. The increased molecular motion in the cooler object manifests as an increase in its temperature. Eventually the molecular kinetic energies equalize as the objects reach the same temperature. According to the second law of thermodynamics, this heat transfer process only occurs spontaneously in one direction – from hot to cold.
Molecular Kinetic Energy
Heat arises from the kinetic energy of molecules. Kinetic energy is the energy of motion. At the microscopic scale, heat is the result of increased molecular motion. As molecules vibrate, rotate, and translate more rapidly within a substance, they bump into each other and transfer kinetic energy during collisions. The higher the average kinetic energy of molecular motion, the higher the temperature of the substance.
Temperature is a direct measurement of the average molecular kinetic energy in a substance. As more heat is added to a system, molecular motion increases, leading to a rise in temperature. The relationship between heat, molecular motion, and temperature is quantified by thermodynamics. But at a conceptual level, increased molecular kinetic energy manifests as increased temperature. This kinetic theory of heat explains how adding thermal energy increases molecular vibrations, rotations, and translations, thereby raising the temperature.
Thermodynamics
Thermodynamics is the branch of physics that deals with heat and temperature and their relation to energy, work, radiation, and properties of matter. The laws of thermodynamics explain the direction and driving mechanisms of heat, work, and other forms of energy transfer. Heat can be understood as an energy transfer between systems due to temperature differences between them according to the laws of thermodynamics. The first law states that the change in internal energy of a closed system is equal to heat supplied to the system minus work done by the system on its surroundings. The second law states that heat will spontaneously transfer from hotter to colder bodies, driving many natural processes including weather, ecosystems, and even the functioning of living things. Understanding heat via the laws and principles of thermodynamics has helped unlock many technologies from steam engines to refrigeration.
Calorimetry
Calorimetry is the process of measuring heat flow and changes. A calorimeter is a device used to make these measurements. Calorimeters allow scientists to quantify the amount of heat absorbed or released during a chemical reaction or physical change.
A simple calorimeter consists of an insulated container filled with a known amount of water or other liquid. A thermometer is used to precisely measure the temperature before and after the process being studied. For example, if a hot metal sample is placed into the calorimeter, the initial and final temperatures would be recorded as the metal cools and heats the water. Using the known heat capacity of the water and the measured temperature change, the amount of energy transferred as heat can be calculated using the equation:
Q = mcΔT
Where Q is the heat energy, m is the mass of water, c is the specific heat capacity of water, and ΔT is the temperature change.
More complex calorimeters allow even more precise measurements. Bomb calorimeters completely surround the reaction vessel to minimize heat loss to the environment. Differential scanning calorimeters can detect tiny heat flows associated with phase changes. Calorimetry has many important applications in chemistry, physics, engineering, and industry.
Everyday Applications
Heat plays an integral role in many everyday processes and technologies that we take for granted. Here are some common examples of how heat concepts manifest in daily life:
Cooking
Cooking relies on the transfer of heat energy to food. As heat is applied through various methods like baking, frying or boiling, it raises the temperature of the food, causing chemical and physical changes that make ingredients more digestible, flavorful and safe to eat.
Engines
Most engines, like those in vehicles and generators, convert heat energy into mechanical work. This occurs through combustion, where a fuel is burned to release thermal energy. This heat creates pressure that pushes pistons and turns a crankshaft to provide power.
Weather
The sun’s radiation heats the atmosphere, oceans and land. Temperature differences create convection currents in the atmosphere and oceans that influence winds, ocean currents and precipitation patterns. Understanding heat concepts helps meteorologists model and predict weather events.
Energy Conservation
Heat is viewed as a conserved quantity according to the first law of thermodynamics. This means that heat cannot be created or destroyed, it can only be transferred between objects or converted into different forms of energy.
The first law of thermodynamics, also known as the law of conservation of energy, states that the change in internal energy of a closed thermodynamic system is equal to the difference between the heat supplied to the system and the amount of work done by the system on its surroundings.
For example, when you rub your hands together, you are converting mechanical work into heat. The kinetic energy of the motion is transformed into thermal energy. The total quantity of energy remains constant, even as the forms change.
Understanding the conservation of heat allows scientists to quantify thermal energy transfers in chemical reactions, biological systems, and mechanical processes. It is a fundamental principle of thermodynamics with broad applications.
Heat Transfer Mechanisms
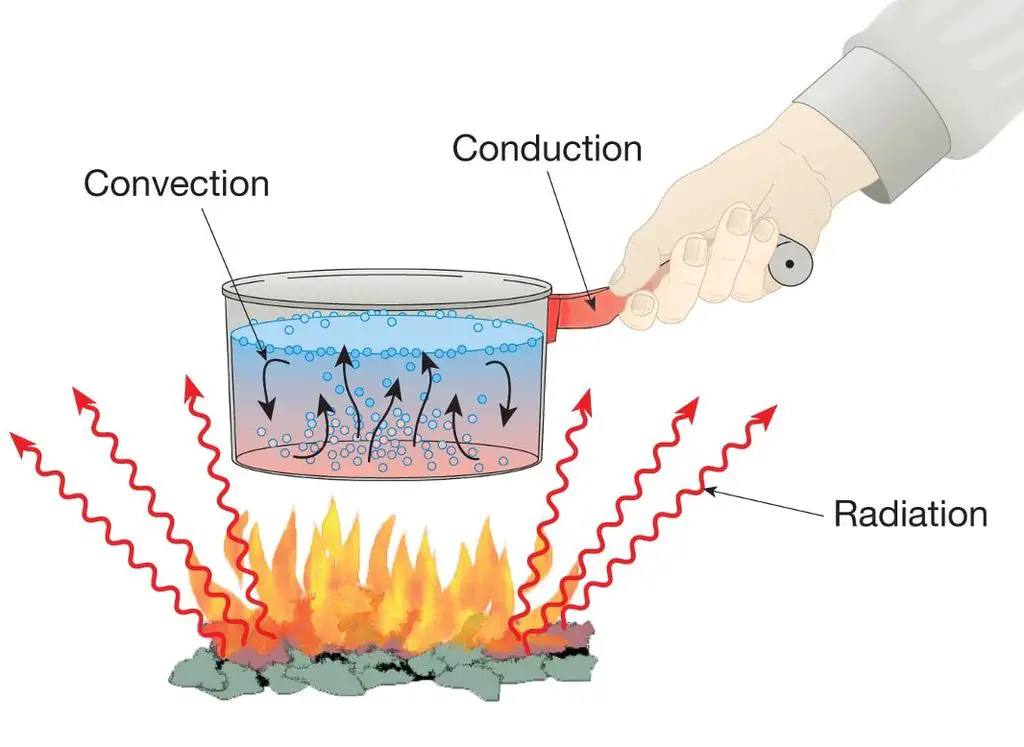
There are three main ways that heat is transferred between objects:
Conduction
Conduction is the transfer of heat between objects that are in direct contact with each other. Heat energy is transferred between molecules within the objects through collisions and interactions at the atomic level. Metals are good conductors of heat because their free electrons can readily absorb and transmit thermal energy.
Convection
Convection is the transfer of heat by the movement of liquids and gases. As a liquid or gas warms, it becomes less dense and rises while cooler material sinks to take its place. This circulation distributes the heat energy. Examples of convection are the air movements that disperse heat from a radiator throughout a room and the ocean currents that transport warm water from the equator towards the poles.
Radiation
Radiation is the transfer of heat via electromagnetic waves directly through space without relying on particle collisions. The sun warms the Earth through radiant energy from nuclear fusion reactions. Similarly, thermal radiation is emitted from any object above absolute zero according to its temperature and emissivity.
Understanding the mechanisms of heat transfer between objects and materials is crucial across science and engineering fields, with key applications in thermodynamics, materials science, and energy engineering.
Quantum Mechanical View
At the microscopic level, heat arises from the random motion and interactions of atoms and molecules. Quantum mechanics provides a statistical description of the energies of particles in matter. The likelihood of finding a particle with a certain energy is distributed according to the Boltzmann distribution. From this distribution, the average thermal energy of particles at a given temperature can be determined.
The more disordered a system is, the higher its entropy. Temperature is a measure of the average energy per particle, whereas entropy is a measure of the overall disorder of the system. Therefore, temperature and entropy are intrinsically linked. As temperature increases, particle motion increases, leading to more dispersed configurations and higher entropy. An object’s internal energy contributes to its temperature according to its degrees of freedom.
Conclusion
In summary, heat is a form of energy that is transferred from one body or system to another as a result of temperature difference. On a molecular level, heat is the kinetic energy and vibrational motion of atoms and molecules. From a thermodynamic perspective, heat flows spontaneously from higher temperature bodies to lower temperature bodies. Measuring heat transfer and changes is an important field known as calorimetry.
Understanding the concept of heat has many everyday applications and is key for energy conservation. The three main mechanisms of heat transfer are conduction, convection and radiation. And from a quantum mechanical view, heat is ultimately the result of increased occupation of higher energy quantum states.
The study and applications of heat are essential across science, engineering and industry. Heat powers engines, allows chemical reactions to occur, and enables efficient energy use in homes and buildings. With rising global temperatures, understanding how to manage heat flow and balance Earth’s energy budget is critical knowledge. Whether it be cooking, electricity generation or weather forecasting, heat and thermal energy are integral to our modern world.