What Is A Chemical Reaction That Produces Electricity?
A chemical reaction is a process where a set of substances (called reactants) are converted into a different set of substances (called products). Chemical reactions involve the breaking and reforming of chemical bonds between atoms, resulting in the transformation of reactants into products.
Electricity generation involves the conversion of other forms of energy into electrical energy. There are several types of chemical reactions that are able to produce electricity. These reactions involve the movement of charged particles like electrons and ions, which generates an electric current that can be harnessed.
Some common types of chemical reactions that can generate electricity include:
- Electrochemical reactions in batteries
- Oxidation of fuels in fuel cells
- Microbial metabolism in bioelectrochemical systems
- Seebeck effect in thermocells
- Piezoelectric effect
- Triboelectric effect
- Pyroelectric effect
- Photovoltaic effect in solar cells
This article will provide an overview of each of these types of electricity-generating chemical reactions.
Batteries
Batteries produce electricity through electrochemical reactions known as oxidation-reduction (redox) reactions. In a battery, chemical energy is converted into electrical energy. A battery contains one or more electrochemical cells, each which contains two electrodes – a positive cathode and negative anode – and an electrolyte solution.
The electrodes are made of different materials, such as lead, zinc, lithium, nickel, and manganese dioxide. The electrolyte carries ions between the electrodes and can be liquid, gel, or solid. When the battery is connected in a circuit, the anode undergoes oxidation, releasing electrons that flow through the external circuit to generate electricity. The cathode is reduced, accepting the electrons from the circuit. The ions move through the electrolyte to balance the flow of electrons and complete the circuit.
Common battery types and their electrode materials include:
- Lead-acid battery – Lead anode, lead dioxide cathode, sulfuric acid electrolyte
- Alkaline battery – Zinc anode, manganese dioxide cathode, potassium hydroxide electrolyte
- Lithium-ion battery – Graphite anode, lithium cobalt oxide cathode, lithium salt electrolyte
- Nickel-cadmium battery – Cadmium anode, nickel oxide cathode, potassium hydroxide electrolyte
Different battery chemistries have advantages and disadvantages in terms of cost, durability, power density, energy density, charging time, safety, and more. Selecting the appropriate battery technology depends on the specific application and requirements.
Fuel Cells
Fuel cells produce electricity through an electrochemical reaction that combines hydrogen and oxygen. At the anode of a fuel cell, hydrogen is oxidized, releasing electrons. At the cathode, oxygen is reduced, consuming electrons. The flow of electrons between the anode and cathode generates electricity. A membrane between the electrodes helps ions flow while keeping the electrons separate to maintain the voltage differential.
There are several types of fuel cells, classified by the electrolyte used:
- PEMFC – Uses a polymer electrolyte membrane, operates at low temperatures around 80°C. Used for vehicles, portable power, and smaller stationary applications.
- SOFC – Uses a solid oxide electrolyte, operates at very high temperatures around 500-1000°C. High efficiency makes it suitable for large stationary power generation.
- PAFC – Uses liquid phosphoric acid as the electrolyte, operates at about 200°C. Used for commercial stationary power generation.
- MCFC – Uses a molten carbonate salt mixture as the electrolyte, operates at 600-700°C. Suitable for natural gas power plants.
- AFC – Uses an alkaline electrolyte like potassium hydroxide, operates below 100°C. Used in space applications.
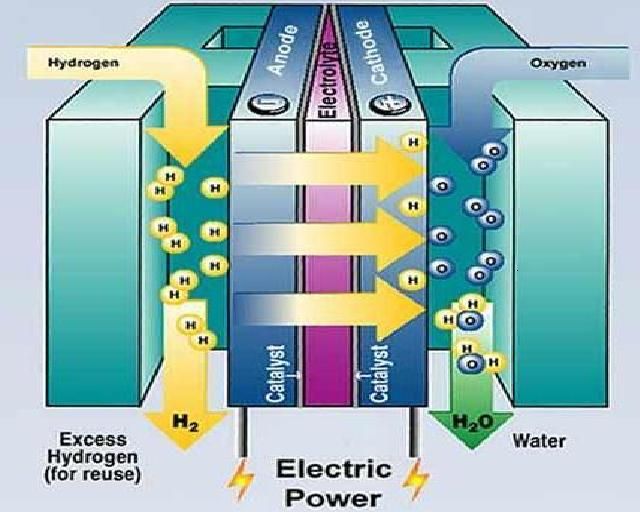
Fuel cells are highly efficient and environmentally friendly since their only byproducts are heat and water. The variety of fuel cell types allows applications at many different scales.
Bioelectrochemical systems
Bioelectrochemical systems are a type of fuel cell that use microorganisms as biocatalysts to drive electrochemical reactions. There are two main types:
Microbial fuel cells (MFCs) – These systems generate electricity from the oxidation of organic matter. In an MFC, bacteria on the anode oxidize compounds like glucose, acetate or wastewater. This generates protons and electrons that travel to the cathode to reduce oxygen. MFCs can generate electricity from a range of organic waste sources, though power densities tend to be low.
Microbial electrolysis cells (MECs) – MECs use electrochemically active microbes to produce hydrogen gas. Organic matter is oxidized at the anode by bacteria. The electrons travel through a circuit to the cathode, where protons and electrons combine with water to produce hydrogen gas. MECs require a small electrical potential to drive the hydrogen evolution reaction.
Key applications of bioelectrochemical systems include:
- Wastewater treatment – MFCs and MECs can oxidize organic contaminants in wastewater while generating electricity or hydrogen.
- Renewable hydrogen production – MECs offer a sustainable way to produce hydrogen gas from organic waste sources.
- Organic waste remediation – These systems can degrade waste from sources like agriculture, food processing and solid waste.
- Biosensors – MFCs can provide rapid detection of substrates like organics, toxins and heavy metals in water.
Overall, bioelectrochemical systems offer an eco-friendly approach for generating electricity, hydrogen and waste treatment services from renewable biomass feedstocks.
Thermocells
Thermocells are devices that convert a temperature difference into electricity using a phenomenon called the Seebeck effect. They consist of two conductors or semiconductors joined together to form a junction or circuit. When one end of the thermocell is heated and the other end is cooled, electrons in the hot end gain energy and move to the cold end, creating an electrical voltage or current flow.
The key component of a thermocell is the thermocouple, made of two different metals or semiconductors. Common materials used include bismuth telluride, lead telluride, silicon-germanium, iron, constantan, and chromel. The larger the temperature difference between the hot and cold junctions, the higher the voltage produced. Even a small temperature gradient of 10-20°C can generate a useful voltage.
Thermocells have been used to produce electricity from waste heat and natural temperature differences in applications like power plants, automobiles, and industry. They can have conversion efficiencies of around 5-8%. While less efficient than other technologies like photovoltaics, thermocells have the advantage of being simple, durable, scalable, and able to work with small temperature differences.
Research is focused on improving the materials and design of thermocouples to increase their efficiency and power output. Thermocells hold promise as a way to capture waste heat and convert it into useful electricity in a solid-state system with no moving parts.
Piezoelectricity
Piezoelectricity refers to the ability of certain materials to generate an electric charge in response to applied mechanical stress. The word piezoelectricity means electricity resulting from pressure. It is an example of a linear electromechanical interaction between the mechanical and electrical state in crystalline materials with no inversion symmetry.
The piezoelectric effect occurs in materials like quartz, Rochelle salt, topaz, tourmaline-group minerals, and some organic compounds such as silk, wood, enamel, bone, DNA, and various proteins. When a mechanical stress or vibration is applied on these materials, the crystalline structure produces a charge separation across the material, generating an electric voltage proportional to the applied force.
Conversely, when a piezoelectric material is subjected to an electric field, it undergoes a small change in dimension. This is known as the inverse piezoelectric effect. Piezoelectric materials exhibit direct and converse piezoelectric effects.
The piezoelectric effect finds useful applications in the production and detection of sound, generation of high voltages, electronic frequency generation, sensors to measure pressure, strain, temperature, acceleration, vibration, or chemical species, and in motors and other actuators. Piezoelectric materials are used for energy harvesting, transforming mechanical energy into electrical energy from ambient sources like footsteps, wind, or vibrations.
Triboelectricity
Triboelectricity is a type of contact electrification that produces static electricity through contact and friction between two different materials. When two materials come into contact, electrons are transferred from one material to the other, resulting in one material becoming positively charged and the other negatively charged. The imbalance of charges between the two materials creates a potential difference, which can be used to generate an electric current if the materials are connected through a circuit.
The triboelectric effect can be used to convert mechanical energy into electrical energy. For example, triboelectric nanogenerators use repetitive contact and separation between two materials to continuously generate electricity. The amount of charge transfer depends on the properties of the materials, their surface roughness, pressure applied, speed of contact, temperature, and other factors. Materials are often optimized and designed in certain patterns to maximize the triboelectric effect.
Triboelectric energy harvesting has applications such as powering small electronic devices, sensors, and smart textiles. Walking or tapping on a triboelectric material can generate enough power through the triboelectric effect. Triboelectric sensors can detect touch, pressure, vibrations, and other mechanical stimuli based on the electrical signals produced through contact. Triboelectricity is considered a promising technology for mechanical energy harvesting, motion detection, and self-powered electronic systems.
Pyroelectricity
Pyroelectricity refers to the ability of certain materials to generate an electric charge in response to changes in temperature. Some crystals, like tourmaline, are pyroelectric – meaning they can convert temperature fluctuations into electricity.
This phenomenon occurs because the atoms in pyroelectric materials lack symmetry, causing the material’s polarization to change with temperature. As the temperature rises and falls, the position of atoms shifts slightly within the crystal structure, altering the electric dipole moment. This leads to a flow of charge across the material, generating an electrical voltage.
Pyroelectricity has useful applications in devices that sense temperature changes, like infrared detectors. The electric signal produced can indicate the presence and strength of infrared radiation. Pyroelectric materials are also used to build sensors for intruder alarms, gas analyzers, laser guidance, and other automation systems. Additionally, waste heat can be converted into electricity through pyroelectric power generation.
Overall, pyroelectricity provides a means of transforming temperature variations directly into electrical energy. This phenomenon in certain crystals and materials can enable temperature sensing and energy harvesting applications.
Photovoltaics
Photovoltaics, also known as solar cells, convert sunlight directly into electricity using the photovoltaic effect. When sunlight hits the solar cell, photons are absorbed by the semiconductor material in the cell, causing electrons to break free from their atomic bonds. This generates pairs of free electrons and electron holes. The built-in electric field in the solar cell separates the electrons and holes before they can recombine, causing the electrons to flow in one direction and the holes in the opposite direction. This flow of electrons and holes is an electric current that can be captured and utilized before being fed back into the cell. Unlike other light-induced chemical reactions that produce an intermediate that then generates electricity, photovoltaics convert light into electricity directly without any intermediate reactions.
Conclusion
There are several chemical reactions that can produce electricity through different processes. Batteries produce electricity through oxidation-reduction reactions. Fuel cells convert the chemical energy from a fuel into electricity through electrochemical reactions. Bioelectrochemical systems use microorganisms to catalyze oxidation-reduction reactions to generate current. Thermocells convert heat into electricity using thermoelectric materials. Piezoelectric materials produce electricity from mechanical stress. Triboelectricity generates charge through friction. Pyroelectricity produces current due to temperature changes in certain materials. Photovoltaics convert light into electricity using the photoelectric effect in semiconductors.
The ability to produce electricity from chemical reactions has enabled the development of portable electronics, electric vehicles, large-scale power generation, and self-powered devices. Ongoing research aims to improve efficiency, reduce costs, and develop more sustainable materials for electrical energy generation via chemical reactions. More work is needed to scale up novel approaches like bioelectrochemical systems. Overall, harnessing chemical reactions for electricity production holds tremendous potential for meeting future energy needs in an increasingly electrified world.