What Is 6 Conservation Of Energy?
The law of conservation of energy states that the total energy in an isolated system remains constant. Energy cannot be created or destroyed, but it can be transformed from one form to another. For example, chemical energy in gasoline can be converted to kinetic energy in a moving car. The total energy in the system (gasoline + car) remains the same.
This principle is considered one of the most fundamental laws in physics and is a key component of the study of thermodynamics. It allows physicists to calculate total system energy and predict how energy will flow between objects and through transformations. Conservation of energy leads to important insights about the universe and forms the basis for many technologies.
In summary, the law of conservation of energy is the foundational tenet that total energy is always conserved, leading to powerful predictive capabilities across many scientific disciplines.
History and Origin
The law of conservation of energy was first proposed in the 1840s by German physicist Julius Robert von Mayer. Mayer arrived at the principle after studying blood circulation and thermodynamics. Around the same time, British physicist James Joule independently discovered the mechanical equivalent of heat, which helped establish the law of conservation of energy.
In 1847, Hermann von Helmholtz published a definitive statement of the law of conservation of energy based on experimental evidence. The law states that the total amount of energy in an isolated system remains constant over time. The law unified observations about heat, mechanical motion, sound, light and electricity under a single theory. It became a fundamental scientific principle and the basis for the theory of thermodynamics.
Formal Statement of Conservation of Energy
The law of conservation of energy states that the total energy of an isolated system remains constant. It is often expressed mathematically as:
ΔE = 0
Where E is the total energy of the system. This means that energy can neither be created nor destroyed, only converted from one form to another. For example, chemical energy in gasoline can be converted to kinetic energy and heat as a car accelerates and moves. The total energy before and after is unchanged.
The law applies to all isolated systems, and is considered one of the fundamental laws of physics. It allows the total energy of a system to be calculated at any given time. The law of conservation of energy is itself a consequence of the translational symmetry of time – that physical laws remain unchanged as time passes.
Applicability
The law of conservation of energy applies to physical systems that are closed and isolated. A closed system means that no matter can enter or exit, while isolated means that no heat and no work can enter or exit. For these types of systems, the total energy remains constant over time.
Some examples where conservation of energy applies include:
- Mechanical systems like pendulums, springs, and frictionless surfaces
- Thermodynamic processes that occur in a closed chamber
- Isolated chemical reactions that do not release heat
The law does not apply to systems that are open or non-isolated. For example, living organisms continuously exchange matter and energy with their surroundings, so they do not adhere to conservation of energy. The Earth also gains energy from the Sun and loses energy to space, so the total energy is not conserved.
On a cosmological scale, conservation of energy does not strictly hold due to factors like the expansion of the universe and dark energy. Overall, the law applies well in closed and isolated systems, but fails in open systems with significant energy exchange.
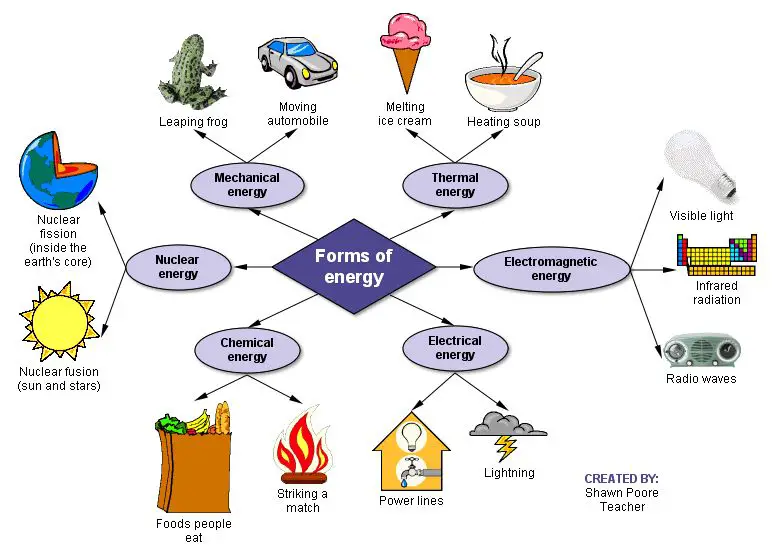
Forms of Energy
There are many different forms of energy that are conserved according to the law of conservation of energy. These include:
-
Mechanical Energy – This includes kinetic energy and potential energy. Kinetic energy is the energy of motion, such as the motion of a ball being thrown. Potential energy is stored energy due to an object’s position or shape, such as a ball held at a height above the ground.
-
Thermal Energy – This is the internal energy of an object due to the motion of its atoms and molecules. It includes both heat and internal energy. Thermal energy is converted between heat and mechanical energy during processes like heating/cooling, phase transitions, and pressure changes.
-
Electrical Energy – The energy from electric fields or the movement of electrons. It includes electrostatic potential energy, as well as the energy in electrical currents.
-
Chemical Energy – The potential energy stored in the atomic bonds between atoms that make up a chemical compound. It can be released during chemical reactions.
-
Nuclear Energy – The energy stored in the nucleus of an atom, which can be released in nuclear reactions. This includes binding energy, as well as rest mass energy.
-
Electromagnetic Radiation – Energy carried by electromagnetic waves, such as radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, x-rays, and gamma rays.
According to the law of conservation of energy, the total amount of these forms of energy in an isolated system remains constant.
Real World Examples
Conservation of energy can be readily observed in our everyday lives. Here are some examples:
Rolling Ball: When a ball rolls down a slope, its potential energy gets converted to kinetic energy. As it rolls up the opposite slope, the kinetic energy gets converted back to potential energy. The total mechanical energy remains the same (if we ignore friction losses).
Pendulum: As a pendulum swings back and forth, there is a continual exchange between kinetic energy and gravitational potential energy. The total energy remains constant through the swing (again, ignoring frictional losses).
Bouncing Ball: When a ball bounces off the floor, kinetic energy gets converted to elastic potential energy as it deforms on impact. This potential energy gets converted back to kinetic as the ball rebounds. Overall energy is conserved in the bounce (excluding heat and sound energy dissipated).
Roller Coaster: The changes in a roller coaster’s kinetic and potential energy as it goes through its course demonstrates conservation of energy. At the top of a hill, it has maximum potential energy, which turns into kinetic energy as it speeds downhill. This kinetic energy gets converted back into potential energy as it climbs the next hill. The total mechanical energy remains constant (neglecting friction).
Common Misconceptions
There are a few common misconceptions people have about the conservation of energy:
1. Energy is “used up” or “runs out.” This is not true – energy can be converted from one form to another, but the total amount of energy in a closed system remains constant. Energy is never created or destroyed.
2. Friction causes energy to disappear. Friction converts kinetic energy into thermal energy in the form of heat. The energy still exists in the system as heat.
3. Objects “use up” their kinetic energy. An object’s kinetic energy can be converted into other forms like potential energy, but the total amount of energy remains the same. The object does not “use up” its own energy.
4. Energy can be destroyed. The first law of thermodynamics states that energy cannot be created or destroyed, only transformed from one state to another. The total amount of energy in a closed system remains constant.
5. Perpetual motion machines can be created. This would violate the conservation of energy, as these imaginary machines would be able to continually output energy without any input. Machines cannot create energy from nothing.
Understanding that energy transforms between different states rather than being used up or destroyed is key to grasping the conservation of energy.
Applications and Importance
The principle of conservation of energy has many important real-world applications in science and engineering. Here are some examples:
Mechanical engineering: Conservation of energy is used to analyze the efficiency and power output of engines and machines. Engineers apply the principle to minimize energy losses and optimize designs.
Thermodynamics: The laws of thermodynamics utilize conservation of energy to study heat transfer, work, and energy transformations in thermal systems. This allows the design of power plants, refrigerators, and heat engines.
Electrical engineering: Conservation of energy is key to analyzing electrical circuits. The total energy in a closed system must remain constant, enabling calculations of current, resistance, and voltage.
Chemical engineering: Energy balances using conservation principles are essential in studying chemical reactions and processes. This helps optimize chemical plant and reactor operations.
Biology: Living organisms require energy to survive, provided by food. Applying conservation laws enables calculating metabolic rates and energy flows through ecosystems.
Astrophysics: The energy released by the sun through nuclear fusion is conserved, powering solar systems. Stellar energy production can be analyzed via conservation principles.
Overall, the conservation of energy is a fundamental scientific concept that underlies many disciplines. It provides a powerful analytical tool with broad relevance across science, engineering, and nature.
Limitations
The law of conservation of energy is considered one of the most fundamental laws in physics. However, it does have some practical limitations in certain situations:
Friction: In processes where friction is present, some energy gets converted into heat. This heat energy can dissipate into the environment, so the total energy is not perfectly conserved.
Inelastic Collisions: When objects collide inelastically and deform or break apart, kinetic energy gets converted into other forms like heat and sound. The dissipation makes it seem like energy disappears.
General Relativity: Einstein’s theory states that energy and mass are equivalent. In systems with very strong gravitational fields, like close to a black hole, energy can transform into mass and vice versa. So the total energy is not strictly constant.
Quantum Mechanics: At very small quantum scales, Heisenberg’s uncertainty principle allows for temporary fluctuations in energy to occur. Total energy is still conserved on average, but not perfectly at exact instants.
New Energy Creation: Some hypothesize that empty space contains energy fields that can create particle-antiparticle pairs spontaneously. If real, this vacuum energy could be an exception to conservation of energy.
Summary
The law of conservation of energy is one of the most fundamental laws in physics. It states that the total energy in an isolated system remains constant – it cannot be created or destroyed, only transformed from one form to another. This law applies across all areas of science and has important real-world applications.
In summary, the key points about the conservation of energy are:
-
Energy cannot be created or destroyed, only converted between different forms.
-
In an isolated system, the total amount of energy remains the same.
-
Forms of energy include kinetic, potential, thermal, chemical, nuclear and others.
-
Examples of energy transformation include generating electricity from heat, photosynthesis converting light to chemical energy, and batteries converting chemical energy to electrical.
-
The conservation of energy explains where energy comes from and goes in processes.
-
While energy can change form, it cannot disappear – this allows us to track energy transfers scientifically.
The law provides a fundamental understanding of the nature of energy and allows us to quantify energy transfers in closed systems. It has many important applications in science and technology.