What Happens To Most Substances When They Are Heated?
Heating substances induces changes by increasing their kinetic energy. The effects of heating depend on the substance being heated and conditions like temperature and pressure. Heating can cause physical changes like melting, evaporation, expansion, as well as chemical changes like decomposition and combustion. The most common effects of heating substances are changes of state from solid to liquid to gas, thermal expansion, decomposition reactions, combustion, and phase changes. This article will provide an overview of what happens when various substances are subjected to heating.
Changes of State
When a substance is heated, it can undergo various changes of state. The most common changes of state are:
Melting
Melting occurs when a solid is heated to its melting point, causing it to transition to a liquid state. The molecules in the solid gain enough energy from the heat that the intermolecular forces holding them in a rigid lattice structure are overcome, allowing the molecules to move freely and take the shape of the container.
Boiling
Boiling occurs when a liquid is heated to its boiling point, causing it to transition to a gaseous state. At the boiling point, the kinetic energy of the liquid molecules is high enough that some molecules can break free from intermolecular attractions and escape as a gas. As more heat is added, more liquid molecules enter the vapor phase.
Evaporation
Evaporation is the gradual transition from liquid to gas that occurs at any temperature below the boiling point. As heat is added to a liquid, kinetic energy causes random molecules near the surface to overcome intermolecular forces and escape as a gas. Evaporation occurs more slowly than boiling.
Sublimation
Some substances transition directly from solid to gas without entering a liquid phase. This process is called sublimation. The added heat gives molecules enough energy to break free and enter the gas phase without melting first. Dry ice (solid CO2) sublimates at room temperature.
Thermal Expansion
One of the most common effects that heating has on substances is expansion. When most substances are heated, the added energy from the heat causes the molecules and atoms to vibrate and move faster and farther apart. This increase in molecular motion pushes the molecules slightly farther apart, causing nearly all substances to expand to some degree.

The amount of expansion depends on the material and temperature change. Solids typically expand the least with heat, while gases expand much more substantially. However, the expansion in solids and liquids can still be noticeable and problematic if not accounted for in engineering and construction. For example, hot asphalt roads will buckle if not allow to properly expand, and railroad tracks can bend on hot days if not pre-stressed to allow for thermal growth.
Metals are particularly prone to noticeable expansion with heat. A hot metal pan on the stove will be slightly larger than the same pan at room temperature. For applications that require high precision, such as machined metal parts, engineers must compensate for thermal growth during heating and contraction during cooling to hit tolerance specifications.
Decomposition
Decomposition is a chemical reaction that occurs when some substances break down into simpler substances when heated. This happens because heating provides energy to break the chemical bonds holding the molecules of the original substance together.
For example, when sugar is heated it decomposes into carbon and water vapor. The sucrose molecules in sugar contain carbon, hydrogen and oxygen atoms bonded together. When heated, these bonds break and the carbon and hydrogen rearrange to form water vapor, while the excess carbon is left behind.
Other common examples of thermal decomposition include calcium carbonate decomposing into calcium oxide and carbon dioxide, and ammonium chloride decomposing into ammonia and hydrogen chloride. Even something as simple as wood breaking down into charcoal and ash when burned is a decomposition reaction.
The temperature at which decomposition occurs depends on the substance. Stronger molecular bonds require more energy (and thus higher temperatures) before the molecules will break apart.
Combustion
Combustion is an exothermic chemical process in which a substance reacts with oxygen and gives off heat. When a flammable substance is heated to its ignition temperature, it can begin to burn and undergo combustion. The ignition temperature, also called the flash point, is the lowest temperature at which a substance emits enough vapor to ignite and combust.
For example, paper has an ignition temperature around 451°F (233°C). If paper is heated to this temperature or higher, such as over an open flame, it will begin to burn. The heat from the flame raises the paper to its flash point, allowing combustion to occur. As the paper combusts, it releases heat and light energy. Combustion will continue as long as fuel and oxygen are present. Removing the heat source or oxygen supply will extinguish the fire.
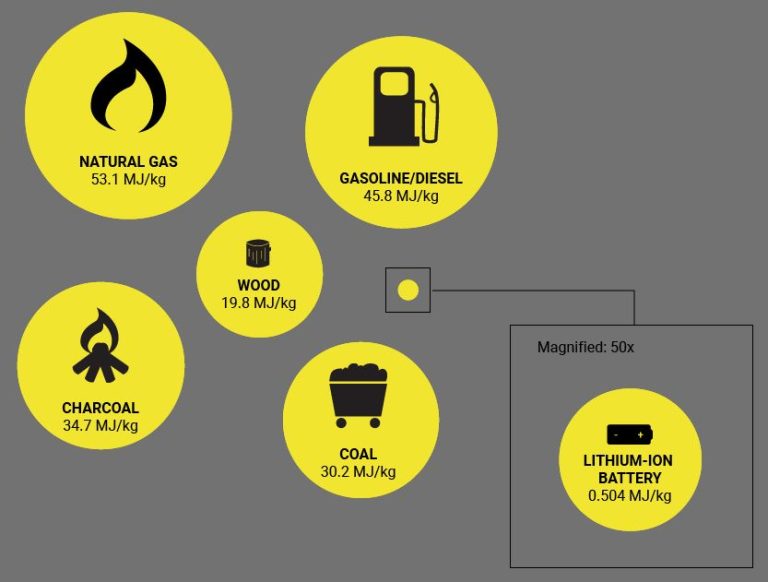
Gasoline, alcohol, natural gas, propane, wood, and coal are other common flammable substances. Each has its own unique ignition temperature at which it will catch fire when heated. Knowing the flash point allows proper handling and storage of combustible materials. Keeping flammable substances away from direct heat sources is important to prevent unwanted combustion events.
Chemical Reactions
Heating substances can cause chemical reactions by providing the energy needed to break bonds between atoms. The breaking and reforming of chemical bonds results in new chemical compounds being formed.
Some examples of chemical reactions induced by heating include:
- Combustion – The reaction between a fuel and oxygen which produces heat and new chemical products like carbon dioxide and water.
- Thermal decomposition – Breaking down of a single compound into simpler substances when heated. For example, calcium carbonate decomposing into calcium oxide and carbon dioxide when heated.
- Oxidation – A reaction where a substance combines with oxygen when heated. For example, the oxidation of metals like iron to form rust.
- Redox reactions – Chemical reactions involving the transfer of electrons between substances. Heating can provide the activation energy needed for these reactions.
- Polymerization – Joining together of simple units called monomers into chains or networks to form polymers. Many polymers are formed through heating chemical monomers.
The input of heat energy allows atoms and molecules to move faster, collide more frequently, and overcome energy barriers to react and form new chemical bonds resulting in different chemical products.
Phase Diagrams
Phase diagrams are graphical representations of the phases of matter under different conditions of temperature and pressure. They allow us to visualize what happens to substances when they are heated or cooled under varying pressures.
A typical phase diagram has temperature on the x-axis and pressure on the y-axis. The lines on the diagram represent phase boundaries – for example, between solid and liquid, or liquid and gas. The point where the lines meet is called the triple point, where all three phases coexist in equilibrium.
As a substance is heated at constant pressure, it will follow a heating path from left to right across the phase diagram. For example, a solid substance first melts into a liquid as it absorbs heat, then the liquid vaporizes into a gas with further heating. The reverse occurs along a cooling path from right to left.
At higher pressures, substances generally require more heat to undergo a phase change. So the phase boundaries shift to higher temperatures as pressure increases. This is why water boils at a higher temperature in a pressure cooker.
Understanding phase diagrams allows scientists to predict and control phase changes of substances during heating and cooling processes. They are essential tools in fields like materials science, chemical engineering, and physics.
Crystallization
When a solution containing a dissolved solid compound is cooled, crystals often begin to form. This process is called crystallization or recrystallization. As a solution cools, the solubility of the solute decreases, causing it to precipitate out of solution as crystalline solids. Temperature plays a key role in crystallization – super-saturated solutions at higher temperatures will readily form crystals as they cool. The formation of crystals can be encouraged by providing surfaces for crystal growth, or by agitating the solution.
Crystallization is commonly used as a purification technique. An impure solid compound is dissolved at elevated temperature, then allowed to slowly cool, enabling purification by precipitation of crystals while impurities remain dissolved. The more slowly the solution cools, the larger the crystals will grow. Large, pure crystals are ideal for analysis techniques like x-ray crystallography to determine molecular structure.
The melting point, or temperature at which a crystalline solid transitions to a liquid state, is a characteristic physical property. Melting points can help identify unknown substances and assess sample purity. The higher the purity, the sharper the melting point range will be. Melting point determination involves heating a sample and identifying the temperature range over which melting is observed. This analysis further demonstrates the strong link between crystallization and how temperature influences the state of matter.
Everyday Examples of Changes from Heating
Heating substances produces observable effects in many aspects of everyday life including cooking, manufacturing, and weather. Here are some examples:
Cooking: Applying heat is fundamental to cooking. It can melt, boil, bake, or roast foods. For example, heating butter melts it from a solid to a liquid. Boiling water turns it into steam. Baking bread allows the dough to rise and obtain a spongy texture. Meat changes color and becomes more tender when roasted in the oven.
Industry: Factories apply heat to reshape metal. Welding melts metal to fuse pieces together. Kilns fire clay at high temperatures to create pottery. Glassblowing uses heat to mold glass into various shapes.
Weather: The sun’s warmth causes water on earth to evaporate and rise into the air as water vapor. This vapor condenses in the atmosphere to form clouds and leads to precipitation.
Conclusion
In summary, heating most substances leads to a variety of changes depending on the material and conditions. The most common effects of heating include:
- Changes of state from solid to liquid to gas as kinetic energy increases.
- Thermal expansion as particles move farther apart.
- Decomposition into simpler substances.
- Combustion with oxygen to form new compounds.
- Acceleration of chemical reactions as energy barriers are overcome.
- Melting, boiling, sublimation, or crystallization as predicted by phase diagrams.
Heating is an important process utilized in many everyday applications to purify, sterilize, extract energy, manufacture materials, and transform substances into more useful forms. Care must be taken with flammable, hazardous, or sensitive compounds. Overall, the effects of heating demonstrate how energy can be used to alter matter at the atomic and molecular levels.