What Are The 1St 2Nd And 3Rd Laws Of Thermodynamics?
Thermodynamics is the branch of physics that deals with heat, work, and temperature, and their relation to energy, radiation, and physical properties of matter. The laws of thermodynamics define fundamental physical quantities like temperature, energy, and entropy and describe how they relate to each other and how they move between a system and its surroundings.
The laws of thermodynamics have wide-reaching applications in science and engineering. For example, they are used extensively in physics and chemistry to study the behavior of gases, liquids, and solids at the molecular level. The laws form a fundamental basis for analyzing the conversion of thermal energy into mechanical work in heat engines and power plants. They are also critical to designing refrigerators, air conditioners, and other devices.
There are four laws of thermodynamics, with the first three being the most important and well-established. The zeroth law defines thermal equilibrium and provides a basis for temperature measurement. The first law expresses the conservation of energy. The second law defines the concept of entropy and provides directionality to natural processes. The third law establishes a minimum entropy value and provides a reference point. Together, these laws provide a broad theoretical framework for analyzing heat, work, and energy transfer processes.
The Zeroth Law of Thermodynamics
The Zeroth Law of Thermodynamics states that if two thermodynamic systems are each in thermal equilibrium with a third system, then they are also in thermal equilibrium with each other. This law helps define the concept of temperature and the transitive property of systems being in thermal equilibrium.
For example, if system A is in thermal equilibrium with system B, and system B is in thermal equilibrium with system C, then according to the Zeroth Law, systems A and C are also in thermal equilibrium with each other. Their temperatures will be equal even if they are not directly connected or interacting.
This law allows the creation of a thermometer, which can measure the temperature of a system by checking for thermal equilibrium against a reference scale. It provides an empirical definition of temperature and a fundamental principle for thermodynamics.
The First Law of Thermodynamics
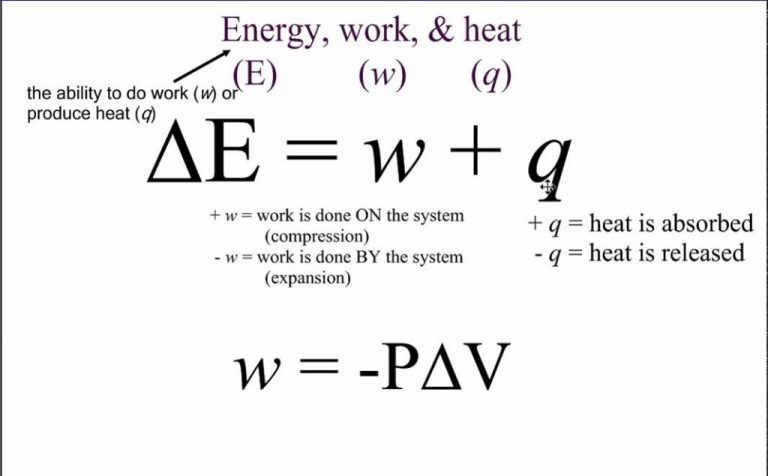
The First Law of Thermodynamics states that the change in internal energy of a thermodynamic system is equal to the heat supplied to the system minus the work done by the system. This is essentially a statement of the conservation of energy – the total energy of an isolated system remains constant. The First Law is expressed by the following equation:
ΔU = Q – W
Where:
- ΔU is the change in internal energy of the system.
- Q is the amount of heat transferred to the system.
- W is the work done by the system on its surroundings.
This shows that the change in internal energy of a closed system is dependent only on the heat added to the system and the work done by the system. If Q is positive, then heat is added to the system. If W is positive, then the system has done work on its surroundings. The First Law encompasses the relation between heat, work and internal energy for thermodynamic processes.
The Second Law of Thermodynamics
The Second Law of Thermodynamics builds on the First Law by introducing the concept of entropy. Entropy is a measure of the disorder or randomness of a system. The Second Law states that the entropy of an isolated system always increases over time. In other words, isolated systems naturally tend towards disorder and randomness.
This law has profound implications, as it suggests that most physical processes are irreversible. The Second Law makes a key distinction between spontaneous and non-spontaneous processes. Spontaneous processes occur naturally without any outside influence. Examples include ice melting, heat flowing from hot to cold, and gas dispersing to fill a container. These processes increase the entropy of the universe.
On the other hand, non-spontaneous processes require some external influence or input of energy to occur. Examples include ice freezing, heat flowing from cold to hot, and gas molecules becoming more concentrated. These processes decrease entropy. Non-spontaneous processes will not happen on their own without added energy.
The Second Law of Thermodynamics sets fundamental limits on what is possible within thermodynamic systems. It shows that while energy is always conserved, it tends to become less usable over time. This law has profound scientific, philosophical, and technological implications.
The Third Law of Thermodynamics
The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero. This law provides an absolute reference point for entropy.
The third law was developed independently by chemist Walther Nernst and physicist Max Planck in the early 1900s. Their statements of the law were slightly different. Nernst’s heat theorem stated that the entropy change for any process approaching zero kelvin is zero. Planck’s statement was that the entropy of a system at absolute zero is a well-defined constant.
These statements are generally combined into the single statement of the third law above. The key conclusions that can be drawn from the third law are:
- Absolute zero cannot be reached through any finite number of processes.
- As temperature approaches zero, entropy approaches a minimum value.
- At absolute zero, the entropy of a perfectly ordered crystal is zero.
The third law provides a foundation for the definition of absolute entropy values. By establishing zero entropy at zero kelvin, it makes the calculation of entropy changes independent of the path or reference states. This allows entropy values to be determined for any thermodynamic system at any temperature.
Examples and Applications
The laws of thermodynamics have many important real-world examples and engineering applications. Here are a few:
Car engines: Internal combustion engines like those in cars rely on the laws of thermodynamics. The burning of gasoline involves chemical reactions that transform the fuel’s energy into heat, pressure, and motion. The engine converts this into useful mechanical work to drive the wheels. But no engine can be 100% efficient due to entropy and the Second Law.
Refrigerators: Refrigerators and air conditioners use cycles of compression and expansion of fluids to absorb heat from one location (the refrigerator interior) and release it into another (typically the surrounding room). This process of cooling relies heavily on the First and Second Laws to function.
Power plants: Fossil fuel and nuclear power plants operate by heating water to produce steam that spins a turbine generator to produce electricity. These thermal cycles, which allow converting heat into work, follow the Laws of Thermodynamics. The limits imposed by the Laws affect the maximum possible efficiency.
Solar cells: Photovoltaic solar cells convert sunlight into electricity. Photons from the sun excite electrons in the solar cell material to higher energy states, allowing an electric current to flow. The excited electrons cannot be fully converted to electricity though, with some energy inevitably lost as heat. This loss process is governed by the Second Law.
Chemical processes: Chemical engineering processes like distillation columns, electrolysis, and catalytic cracking rely on manipulation of heat and energy flows. Careful design using thermodynamic principles and accounting for entropy constraints is key to achieving high efficiency and yields.
Relation to Statistical Mechanics
The laws of thermodynamics have a deep connection with statistical mechanics, which describes the average behavior of large numbers of microscopic particles. While thermodynamics focuses on macroscopic properties like temperature and pressure, statistical mechanics provides a microscopic, molecular interpretation of these concepts.
For example, temperature is related to the average kinetic energy of molecules. Pressure is explained by the momentum transfer of molecules colliding with surfaces. The second law of thermodynamics, which states that entropy always increases in an isolated system, is a statistical consequence of the huge number of possible microstates that lead to macroscopic disorder.
Ludwig Boltzmann showed that entropy could be defined in terms of the number of possible microscopic arrangements of a system with given macroscopic properties. Entropy increases because there are more disordered states than ordered ones. The probability of a system moving to a higher entropy state is simply a statistical effect.
Overall, statistical mechanics reveals how the orderly rules of thermodynamics emerge from the random chaos of molecular interactions. The laws of thermodynamics are empirical observations about large systems, while statistical mechanics provides a theoretical framework connecting the microscopic to the macroscopic.
Limitations
While the laws of thermodynamics provide fundamental explanations for the behavior of energy and matter, they do have limits in their explanatory power. Some key limitations include:
They do not account for quantum effects. The laws of thermodynamics function well in the macroscopic realm but break down when applied at the microscopic, quantum scale. Phenomena like entanglement are not captured by classical thermodynamics.
They do not explain the directionality of time and increasing disorder. While the second law describes that closed systems tend towards higher entropy over time, it does not explain why time has a direction or why disorder tends to increase.
They do not determine the microscopic properties of a system. While the laws describe the overall behavior of a system’s energy, entropy, heat transfer etc., they do not specify the precise positions and properties of the underlying microscopic particles. Statistical mechanics is needed to derive specific microscopic details.
They cannot predict the future states of a system. The laws describe possible states and constraints but cannot predict the precise path a thermodynamic system will take over time. Initial conditions also influence outcomes.
They break down at or near singularities. When dealing with infinities and singularities like black holes, the known laws of thermodynamics encounter inconsistencies and mathematical issues. A complete theory of quantum gravity is needed to resolve such singularities.
Overall, while extraordinarily useful, the classical laws of thermodynamics have boundaries in their explanatory scope. They need to be combined with other disciplines like quantum theory, relativity, cosmology etc. to provide a complete description of physical reality. New physics may be needed to fully unite thermodynamics with these other fields.
Frequently Asked Questions
Here are some common questions and answers about the laws of thermodynamics:
What is the difference between the first and second laws of thermodynamics?
The first law states that energy can neither be created nor destroyed in an isolated system. It is concerned with the conservation of energy. The second law states that the entropy of an isolated system always increases. It is concerned with the direction of processes and heat transfer.
What does the third law of thermodynamics state?
The third law states that the entropy of a perfect crystal at absolute zero is exactly equal to zero. This provides an absolute reference point for entropy.
Why can’t you win at thermodynamics?
You can’t win at thermodynamics because the second law states that for any process, the total entropy of the universe increases. This means usable energy is always being lost to heat and friction. Perpetual motion machines are impossible.
What is perpetual motion and why is it impossible?
Perpetual motion is the hypothetical ability of a closed system to operate continuously without any external source of energy. It is impossible because it would violate the first or second law of thermodynamics. Machines cannot recycle the same energy infinitely without losing some to heat.
How are thermodynamics and statistical mechanics related?
Statistical mechanics provides a microscopic explanation of thermodynamic behavior in terms of molecular motion and energy. It uses probability and statistics to link the properties of individual molecules to bulk thermodynamic quantities.
Conclusion
The three laws of thermodynamics provide the foundation for understanding heat, energy, and entropy in physics and chemistry. They describe important limits and behaviors of energy and matter that are essential to science and technology.
The first law of thermodynamics, the law of conservation of energy, states that energy cannot be created or destroyed, only transformed from one form to another. This law provides the basis for understanding all energy transfers and transformations.
The second law of thermodynamics describes how entropy, a measure of disorder, always increases in isolated systems. This law explains why some processes occur spontaneously while their reverse processes do not. It also establishes the fundamental upper limit on efficiency for heat engines and many energy transformations.
The third law of thermodynamics defines the lowest possible temperature, called absolute zero. This law helps explain the behavior of matter as it approaches absolute zero. It also allows calculation of absolute entropy values, which is useful for predicting the spontaneity of certain chemical reactions.
Together, these three fundamental laws provide deep physical insights and mathematical formulas to analyze thermal systems. They form the foundation of thermodynamics and have profoundly influenced physics, chemistry, engineering, and other fields. Their discoveries marked major milestones in humanity’s understanding of the universe.