What Are Examples Of Chemical Energy To Electrical?
Chemical energy is the potential energy stored in the bonds between atoms that make up molecules. It is energy stored within a substance that can be converted into other forms of energy when chemical reactions occur. Electrical energy is the energy carried by moving electrons in an electric current. Converting between chemical energy and electrical energy involves facilitating chemical reactions that generate electron flow or using electrons to drive chemical reactions.
There are several ways to convert chemical energy into electrical energy. Some common methods include electrochemical reactions in batteries and fuel cells, harnessing biochemical reactions, thermoelectric generators, piezoelectric materials, static electricity, and electrochemistry techniques. This article will provide an overview of these key examples of transforming chemical energy to electricity.
Chemical Batteries
Chemical batteries convert the chemical energy stored in their active materials directly into electrical energy through electrochemical oxidation-reduction reactions. These reactions involve the transfer of electrons from one material to another, producing an electric current.
The most common examples of chemical batteries are:
- Alkaline batteries – These contain zinc and manganese dioxide electrodes with an alkaline electrolyte. The zinc oxidizes, releasing electrons that travel through the external circuit. The manganese dioxide is reduced at the cathode as it accepts the electrons.
- Lithium-ion batteries – These contain lithium ions that move between the anode and cathode. At the anode, lithium atoms release electrons that travel through the external circuit. The lithium ions then migrate through the electrolyte and are inserted into the cathode material.
This electrochemical reaction continues generating current until the battery’s chemical reactants are consumed and no more electrons can be released.
Fuel Cells
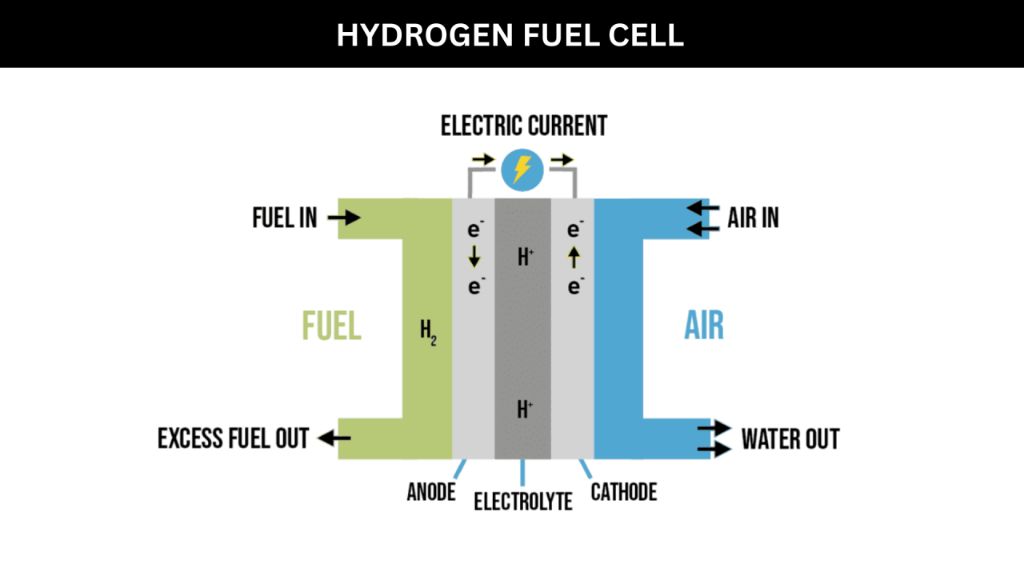
Fuel cells are electrochemical devices that convert the chemical energy from a fuel into electricity through a chemical reaction. They generate electricity continuously as long as fuel and oxidant are supplied.
In a fuel cell, the fuel (such as hydrogen) is oxidized at the anode, releasing electrons that travel through an external circuit to generate electricity. At the cathode, oxidant is reduced, often using oxygen from air. The electrons travel from the anode to the cathode through the external circuit, producing an electrical current.
Some examples of fuel cells include:
- Hydrogen fuel cells – These use pure hydrogen gas as a fuel. The chemical reaction combines hydrogen and oxygen to produce electricity, with water and heat as byproducts.
- Methanol fuel cells – Also called direct methanol fuel cells, these use methanol as a fuel. The methanol is directly oxidized at the anode.
- Solid oxide fuel cells – These use a solid oxide electrolyte and operate at very high temperatures. They can utilize various fuels like hydrogen, carbon monoxide, natural gas, etc.
Fuel cells are highly efficient at extracting energy from fuel and have very low emissions. This makes them a promising technology for power generation in vehicles, buildings, and other applications.
Biochemical Reactions
Biochemical reactions that occur within living organisms can be harnessed to generate electricity. One example is microbial fuel cells, which use bacteria to oxidize organic matter and generate current. The bacteria feed on compounds like glucose, acetate or wastewater and transfer electrons to an anode. The electrons then flow through a circuit to the cathode, generating electricity.
Other biochemical reactions used to produce electricity include:
- Enzymatic biofuel cells – These use enzymes as the catalyst to oxidize fuels like glucose and generate current.
- Photosynthesis – Chloroplasts in plants are able to convert sunlight into chemical energy through photosynthesis. This can be harnessed to generate electricity.
- Compost power – Organic matter decomposing in compost piles produces methane gas. This gas can be collected and used to power electric generators.
Radioisotope Thermoelectric Generators
Radioisotope thermoelectric generators (RTGs) convert heat from the natural radioactive decay of radioactive isotopes like plutonium-238 into electricity using the thermoelectric effect. The heat from the radioactive decay is captured and converted into electrical current by thermocouples made of semiconductor materials.
RTGs have been used extensively to power spacecraft on missions where solar power is not practical. For example, the Voyager 1 and Voyager 2 spacecraft, launched in 1977 to study the outer solar system, were both powered by RTGs since solar power was not feasible far from the Sun. The Cassini spacecraft that orbited Saturn from 2004-2017 was also powered by RTGs.
RTGs are a reliable long-term source of electrical power for satellites and unmanned remote facilities like lighthouses and weather stations. For instance, the US Navy has used RTGs to provide power for remote radar stations located in isolated areas. They can continuously generate electricity for decades, making them ideal for these types of applications.
Piezoelectricity
Piezoelectricity is the ability of certain materials to generate an electrical charge in response to applied mechanical stress. The word piezoelectricity means electricity resulting from pressure. It is an example of converting mechanical energy into electrical energy.
Some naturally piezoelectric materials include bone, DNA, quartz, and some synthetic ceramics like lead zirconate titanate. When a mechanical stress or vibration causes deformation in the atomic structure of these materials, the displacement of the ions produces a voltage across the material.
Piezoelectricity has useful applications in transducers that can convert pressure, acceleration, temperature, strain or force into an electrical signal. Some examples include:
- Piezoelectric sensors – These can detect changes in pressure, acceleration, temperature, strain or force by converting them into an electrical charge.
- Piezoelectric igniters – They use piezoelectricity to generate a spark for ignition in lighters and stoves.
- Piezoelectric actuators – They convert an applied voltage into mechanical motion or force.
Piezoelectric materials make it possible to harvest ambient mechanical energy from vibrations, motion, wind and more to produce electrical energy that can power small electronics and sensors.
Thermoelectric Effect
The thermoelectric effect is a phenomenon where temperature differences can create an electric voltage or current. This works through the thermoelectric properties of certain materials that generate a voltage when exposed to a temperature gradient. The effect can be used to generate electricity directly from heat through a process called the Seebeck effect.
Thermoelectric generators (TEGs) make use of this by employing semiconductor materials, like bismuth telluride, that produce a voltage when one side is heated compared to the other side. The voltage generated can provide a power source directly from a heat source. TEGs are used in applications like converting waste heat into electricity and as power generators for spacecraft.
Thermocouples also rely on the thermoelectric effect. They are made from two dissimilar conductors that are joined at two junctions. When the two junctions are different temperatures, a voltage is produced that relates to the temperature difference. This allows thermocouples to measure temperature by correlating the voltage to known temperature data. They have uses ranging from laboratory instrumentation to appliances and power generation.
In both TEGs and thermocouples, the thermoelectric effect enables generating electricity directly and passively from temperature gradients. The magnitude of electricity produced depends on the materials and temperature difference.
Static Electricity
Static electricity is produced through the friction of two objects rubbing against each other. This transfers electrons from one object to the other, causing them to become electrically charged. The buildup of this charge differential leads to static electricity. When the threshold is reached, discharge occurs in the form of static shocks or sparks.
A common example is when we walk across a carpet in socks and touch a metal doorknob, receiving a zap. The friction between the socks and carpet causes electrons to transfer to our body, charging us up. The doorknob provides a path to ground, creating the shock. Other instances are getting shocks from clothing, electronics, or even just shuffling feet on the floor. Static charge can reach up to 35,000 volts!
On a larger scale, lightning is a powerful discharge of static electricity generated through air friction. Within thunderclouds, air turbulence causes positive and negative electrical charge separation. When the built up charge becomes too high, lightning is released to equalize it. The visible flash we see is the intense static discharge jumping from cloud to cloud or between the ground and clouds.
Triboelectric nanogenerators (TENGs) utilize static electricity for energy harvesting. They are made of materials like
teflon and aluminum that become oppositely charged when contact is made between their surfaces. As they rub together from external motion like vibrations, waves, or rotation, electrons flow back and forth between the materials, inducing electrical current. The generated power can be stored and used to drive small electronics.
Electrochemistry
Electrochemistry involves using electrochemical reactions to produce electricity. One example is electrolysis, which uses electricity to drive a non-spontaneous chemical reaction. Electrolysis involves using an electric current to split chemical compounds into their elements. For example, water can be split into hydrogen and oxygen gas through electrolysis. The electrolysis process works because electrical energy gets converted into chemical energy in the separated elements.
Electroplating and electrorefining metals are other electrochemical processes that can produce electricity as a byproduct. In electroplating, a metal ion solution is reduced onto a conductive surface. As metal ions are deposited, electrons flow through the circuit, generating an electric current. Electrorefining is used to purify impure metals. An electric current passes through a solution with the impure metal, causing pure metal to deposit on the cathode while impurities remain in the solution.
Conclusion
There are many different ways that chemical energy gets converted into electrical energy that we utilize every day. The most common examples are batteries and fuel cells. Batteries convert the chemical energy in reactants like zinc and manganese dioxide into electrical energy through electrochemical reactions. Fuel cells use chemical energy from fuel sources like hydrogen or natural gas to generate electricity through a catalytic process. Biochemical reactions in living organisms also generate electrical impulses like in nerves and muscles. Radioisotope thermoelectric generators use heat from the decay of radioactive isotopes to produce electricity. Piezoelectric materials can create charge when mechanical stress is applied. The Seebeck effect converts heat directly into electricity through temperature differences. Even static electricity from the contact and separation of materials converts the chemical bond energy into electrical energy. Overall, the wide range of examples, from batteries in our devices to static electricity from walking across a carpet, demonstrate the multitude of ways chemical energy gets converted to electricity in our modern lives.