What Are 3 Chemical Changes That Happen In The World?
Combustion
Combustion is a high-energy chemical reaction in which a fuel is oxidized and converted into a different chemical form. Combustion requires an oxidizer, usually oxygen, and a source of ignition to initiate the reaction. The reactants, typically a hydrocarbon fuel, react with oxygen to form carbon dioxide, water vapor, and energy. The energy released enables combustion reactions to be highly exothermic, meaning they give off heat. Some common examples of combustion reactions include burning wood, candles, or natural gas in stoves and heaters.
During combustion, the hydrocarbon fuel breaks down and reacts with oxygen to generate heat and new chemical products. For example, when wood burns, the cellulose and lignin in the wood react with oxygen to form carbon dioxide, water, ash, and energy. Combustion reactions require oxygen to proceed at a rapid rate – without oxygen, fuels smolder slowly instead of burning quickly. Most combustion reactions on Earth occur in an oxygen-rich atmosphere that enables efficient oxidation. The energy released enables combustion reactions like burning wood or gas to maintain their self-sustaining reactions and provide useful heat energy.
Rusting
Rusting is a common chemical change that occurs when iron is exposed to oxygen and moisture. This process is known as oxidation, whereby the iron reacts with oxygen to form iron oxide, which we recognize as rust. Some key things to know about rusting:
Rusting occurs readily when iron, oxygen, and water are present. The chemical reaction can be summarized as:
Iron + Oxygen + Water -> Iron Oxide (Rust)
Examples of rusting are easy to find in everyday life. Cars, metal pipes, bridges, nails, and iron railings are all susceptible to rusting when exposed to air and moisture over time.
Since rusting gradually weakens iron, preventing or slowing rust formation is often desirable. Common prevention methods include galvanization, painting, oiling, and keeping iron dry. Stainless steel alloys also resist rust through the addition of metals like chromium.
Food spoilage
Food spoilage refers to the deterioration of food that makes it undesirable or unsafe to eat. Spoilage is caused by enzymes that are naturally present in foods, oxidation reactions with oxygen, and microbes such as bacteria, molds, and yeasts.
Enzymes in foods continue acting after harvest to break down components like proteins, fats, and carbohydrates. Exposure to oxygen leads to oxidation that degrades nutrients, flavor, and color. Microbes feed on food components, producing byproducts that lead to rotting textures, bad odors and flavors.
Visible signs of spoilage include mold growth, sliminess, discoloration and an unpleasant smell or taste. Spoiled foods provide favorable conditions for potentially dangerous microbes like salmonella, E. coli, and Clostridium botulinum to grow. This can lead to foodborne illnesses if contaminated items are consumed.
Proper food handling, storage at low temperatures, and preservation techniques like canning, freeze drying, and pasteurization help slow down spoilage processes and prevent food safety issues.
Photosynthesis
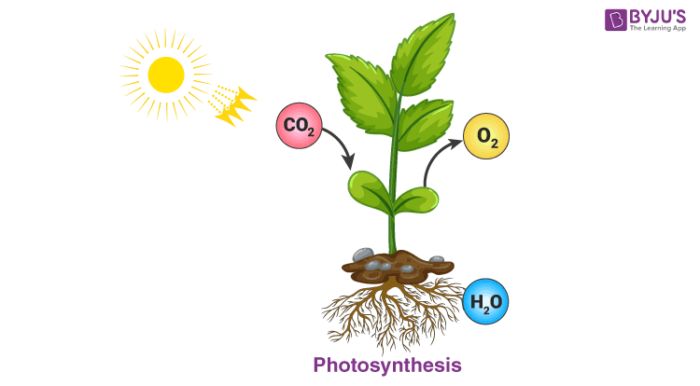
Photosynthesis is the process by which plants and some bacteria produce glucose and oxygen from carbon dioxide and water using energy from sunlight. This chemical process is vital for life on Earth as it provides the glucose that plants need for energy and serves as the foundation for ecosystem food webs. Additionally, the oxygen produced helps maintain atmospheric oxygen levels to support aerobic life.
The overall chemical equation for photosynthesis is:
6CO2 + 6H2O + Light Energy → C6H12O6 + 6O2
This means that for every 6 molecules of carbon dioxide (CO2) and 6 molecules of water (H2O) that are consumed, 1 molecule of glucose (C6H12O6) and 6 molecules of oxygen (O2) are produced. The glucose provides food for plants, while the oxygen is released into the air.
Photosynthesis takes place in chloroplasts, specialized organelles found in plant cells and some algae. Chlorophyll in the chloroplasts captures light energy from the sun to power the reactions. This process converts the sun’s light energy into chemical energy that can be used by living organisms. Without photosynthesis producing oxygen and organic compounds from carbon dioxide, life as we know it on Earth would not exist.
Digestion
Digestion is the process of breaking down food and liquids into molecules small enough to be absorbed and used by the body. It begins in the mouth where food is chewed and mixed with saliva containing enzymes that start breaking down carbohydrates. The food then moves to the stomach where it is mixed with gastric juices containing acids and more enzymes that continue digestion. The acids help break down food while the enzymes break down proteins, fats and complex carbs. The partially digested food moves into the small intestine where it mixes with digestive juices from the pancreas, liver and intestinal walls. More enzymes break down food into nutrients like amino acids, glucose, glycerol and fatty acids while the intestines absorb these nutrients into the bloodstream. Water and minerals are also absorbed. Any undigested material moves into the large intestine where more water is absorbed, leaving only waste matter to be eliminated from the body. Digestion allows the body to acquire the nutrients it needs from the food we consume.
Fermentation
Fermentation is an anaerobic process that converts sugar into carbon dioxide and ethanol or organic acids. Unlike most chemical reactions, fermentation does not require oxygen. Instead, it relies on anaerobic organisms like yeast or bacteria to metabolize sugars and produce these byproducts.
The most common examples of fermentation are the production of beer and wine. For instance, brewer’s yeast metabolizes the sugars derived from barley malt into ethanol and carbon dioxide, creating the alcohol content and carbonation in beer. With wine, yeasts convert the natural sugars in grape juice into ethanol, carbon dioxide, and other flavor compounds.
Fermentation is also used in the rising of bread dough. Yeast metabolizes the sugars present in the dough, generating carbon dioxide gas. This gas gets trapped in the glutens of the dough, causing it to inflate into the characteristic shape of bread.
Beyond alcohol production, fermentation has other industrial applications like producing acids, gases, and certain micronutrients. Overall, fermentation is a key anaerobic process that converts sugars into other compounds, which can then be used in foods, beverages, and commercial products.
Decomposition
Decomposition is the process by which dead organic materials such as plants, food scraps, and animal carcasses are broken down by bacteria, fungi and other decomposer organisms. This releases nutrients back into the soil and atmosphere that can be used by other organisms.
The decomposers that carry out this process include bacteria, fungi, invertebrates like insects and earthworms, and other microorganisms. These organisms consume and digest the dead matter, breaking it down into simpler organic and inorganic compounds.
Decomposition occurs more quickly in warm, moist conditions that allow the decomposers to thrive. Environments with adequate oxygen also promote faster decomposition. The rate of decomposition is affected by factors like temperature, moisture, oxygen levels, and the type of material being broken down.
This nutrient recycling supports natural ecosystems. Decomposition releases carbon dioxide, water, and energy, while recycling nitrogen, phosphorus and other elements back into the soil for reuse by plants. It breaks down waste and cleans up the environment.
Acid-base reactions
Acid-base reactions are chemical reactions that involve the transfer of protons (H+ ions) between an acid and a base. Acids are proton donors while bases are proton acceptors. When acids and bases react, the acid donates protons to the base, resulting in the formation of a salt and water.
A common example of an acid-base reaction is mixing vinegar (acetic acid) and baking soda (sodium bicarbonate). Vinegar contains acetic acid molecules (CH3COOH) that can donate protons, while baking soda contains bicarbonate ions (HCO3-) that can accept protons. When they react, the H+ ions from acetic acid are transferred to bicarbonate, forming carbonic acid (H2CO3) which quickly decomposes into carbon dioxide and water. Sodium ions (Na+) and acetate ions (CH3COO-) are left over, forming sodium acetate salt.
2CH3COOH + NaHCO3 → CH3COONa + H2CO3 → CH3COONa + CO2 + H2O
The fizzing and bubbling is caused by the carbon dioxide gas being released during the reaction.
Acid-base reactions also cause a measurable change in the pH of the solution. Adding an acid like vinegar causes the pH to decrease as more protons are formed, making the solution more acidic. Adding a base like baking soda causes the pH to increase as more protons are removed, making the solution more basic.
Electrolysis
Electrolysis is a chemical reaction that uses an electric current to split compounds. When electricity is applied to a compound, the bonds between the atoms break, separating the elements. This process allows chemists to isolate elements that normally only occur in compounds.
In electrolysis, the compound to be decomposed is liquefied or dissolved and made part of an electrolytic cell. Positively charged cations migrate to the cathode, where they gain electrons and are reduced. Negatively charged anions migrate to the anode, where they lose electrons and are oxidized.
Electrolysis has many industrial applications. It’s used to purify metals like aluminum and copper that can be corroded by impurities. Chlorine gas, used for bleaching and disinfecting, is also produced via electrolysis. The hydrogen needed for artificial fertilizer is obtained using electrolysis of water. Electroplating relies on electrolysis to coat objects with a thin layer of metal.
Conclusion
The world around us is constantly undergoing chemical changes that are essential to life. We summarized some of the most important chemical changes like combustion that provides energy, rusting that breaks down iron, food spoilage that recycles organic matter, photosynthesis that converts carbon dioxide to oxygen, digestion that breaks down food for nutrients, fermentation that produces alcohol, decomposition that returns nutrients to the soil, acid-base reactions that balance pH, and electrolysis that separates elements.
These diverse chemical changes nourish ecosystems, support the climate, sustain human activities, and maintain balanced nutrient cycles. As our world advances, greater knowledge of chemical changes allows us to find cleaner energy sources, reduce pollution, create more efficient industries, produce healthier foods, and improve medicine. With a deeper understanding of the chemical transformations happening around us every day, we can build a more sustainable future.