Is The Conservation Of Energy From One Form To Another?
Defining Energy Conservation
In physics, energy conservation refers to the principle that the total energy in an isolated system remains constant. Energy cannot be created or destroyed, but only transformed from one form into another. For example, a swinging pendulum converts potential energy into kinetic energy and back again over the course of its swing. The conservation of energy is a fundamental concept in physics that follows from the law of conservation of mass and the first law of thermodynamics.
More specifically, energy conservation states that when energy is transferred from one system to another, the quantity of energy before transfer equals the quantity after transfer. While energy can change forms, the total amount of energy in the universe remains fixed. This is true regardless of the type of energy or how it is transformed. Energy conservation is one of the most important laws governing the interactions between matter and energy.
Forms of Energy
There are many different forms of energy that exist in the universe. Here are some of the main forms of energy:
- Thermal Energy – The energy that comes from the vibration and movement of atoms and molecules. Thermal energy relates to the temperature of matter.
- Radiant Energy – The energy carried by electromagnetic radiation, like visible light, ultraviolet light, infrared radiation, radio waves, etc.
- Kinetic Energy – The energy possessed by an object or particle in motion. The faster an object moves, the more kinetic energy it possesses.
- Potential Energy – Stored energy based on an object’s position or arrangement. For example, the potential energy of water held behind a dam.
- Chemical Energy – The energy stored in the bonds between atoms and molecules. Chemical energy can be released in chemical reactions.
- Nuclear Energy – The energy stored within an atom’s nucleus and released in nuclear reactions. Nuclear energy comes from the conversion of mass into energy.
The First Law of Thermodynamics
The First Law of Thermodynamics is a basic scientific law of physics that states that energy cannot be created or destroyed – it can only be transformed from one form to another. This implies that the total quantity of energy in an isolated system always remains constant. This law is important to understanding energy conservation.
The First Law establishes that in any process, the total energy of the universe remains the same. This means that while energy may change forms, the total amount of energy never changes. For example, electrical energy can be converted into light and heat energy, but the total amount of energy remains the same before and after the conversion.
The First Law shows that energy cannot be created out of nothing, nor can it be destroyed into nothing. It can only change its form. This helps explain why energy conservation is possible – because according to the First Law, the total amount of energy is always conserved, even as energy transforms into different types.
Energy Conversion Examples
Energy is constantly being converted from one form to another in the natural world and through human systems. Here are some common examples of energy conversion:
Chemical to Thermal Energy
When wood burns, the chemical energy stored in the wood’s molecular bonds is released as thermal energy and light.
Electrical to Light Energy
Light bulbs convert electrical energy to light energy and thermal energy.
Mechanical to Electrical Energy
Generators convert the mechanical energy of spinning turbine blades into electrical energy.
Nuclear to Thermal Energy
In nuclear power plants, nuclear energy from fission reactions is converted into thermal energy, which is used to boil water into steam that spins turbines to generate electricity.
Solar to Electrical Energy
Solar photovoltaic panels convert photons from sunlight directly into electrical current.
Chemical to Mechanical Energy
In internal combustion engines, the chemical energy stored in fuel is converted into mechanical energy to drive pistons.
Is All Energy Conserved?
While the first law of thermodynamics states that energy can neither be created nor destroyed within a closed system, in real-world scenarios there are often small amounts of energy “lost” or dissipated in the process of transferring energy from one form to another. This is due to inefficiencies and irreversibilities in energy conversions.
For example, when converting chemical energy in gasoline to kinetic energy in a car engine, some energy is lost as heat during the combustion process due to friction and non-ideal gas behavior. Additionally, when charging batteries, there are energy losses through heat dissipation during the charging process. Even when transmitting electricity along power lines, some energy is lost due to resistance in the wires.
While the total amount of energy in a perfectly closed system remains constant, in practical real-world systems, 100% efficiency is impossible to achieve. There will always be some waste heat and energy dissipation. The second law of thermodynamics accounts for these irreversibilities and limitations. So while energy can theoretically be conserved according to the first law, the second law recognizes that some usable energy is always lost in any real process.
Real World Energy Loss
While the first law of thermodynamics states that energy can neither be created nor destroyed, merely transformed, real world systems experience energy loss in practice. This is because no energy transformation is completely efficient, and some waste heat is always generated as a byproduct.
For example, when chemical energy stored in gasoline is converted into kinetic energy that powers a car engine, a significant amount of the initial energy escapes as heat through friction, sound, exhaust fumes, and radiation. Up to 70% of the gasoline’s energy may be lost this way rather than fully converted into useful mechanical work.
Similar inefficiencies occur in other energy conversions like turning nuclear energy into electricity in a power plant, where roughly two-thirds of the initial energy is lost as waste heat. Even charging a battery results in energy leakage, with around 20% dissipating as heat rather than being stored.
These inevitable energy losses are explained by the second law of thermodynamics, which states that entropy or disorder increases in any closed system. The released heat represents greater randomness and disorder at the molecular level. So in practice, some energy is always “degraded” rather than conserved perfectly.
Energy Efficiency
Improving energy efficiency is one of the most effective ways to reduce energy waste and minimize overall energy consumption. Energy efficiency refers to using less energy to perform the same task or produce the same amount of work. For example, replacing an old incandescent light bulb with an LED bulb that uses a fraction of the electricity to produce the same amount of light is an energy efficiency upgrade.
There are many ways to improve energy efficiency both on an industrial level and in our daily lives. Using more efficient manufacturing processes, equipment, appliances, electronics, vehicles, and building techniques conserves energy wasted through transmission, idling, friction, and excessive power usage. Installing better insulation, weatherstripping, energy-saving windows, and programmable thermostats reduces wasted energy in residential and commercial buildings. Simply turning off lights and electronics when not in use also prevents needless energy drain.
Government standards and regulations that mandate more stringent energy efficiency for various products and processes have also driven improvements over the years. Utility companies and government agencies often provide rebates and tax incentives to further motivate the adoption of energy efficient upgrades by businesses and homeowners. Overall, a widespread societal focus on reducing energy waste through improved efficiency is key to minimizing the amount of energy we must produce and consume.
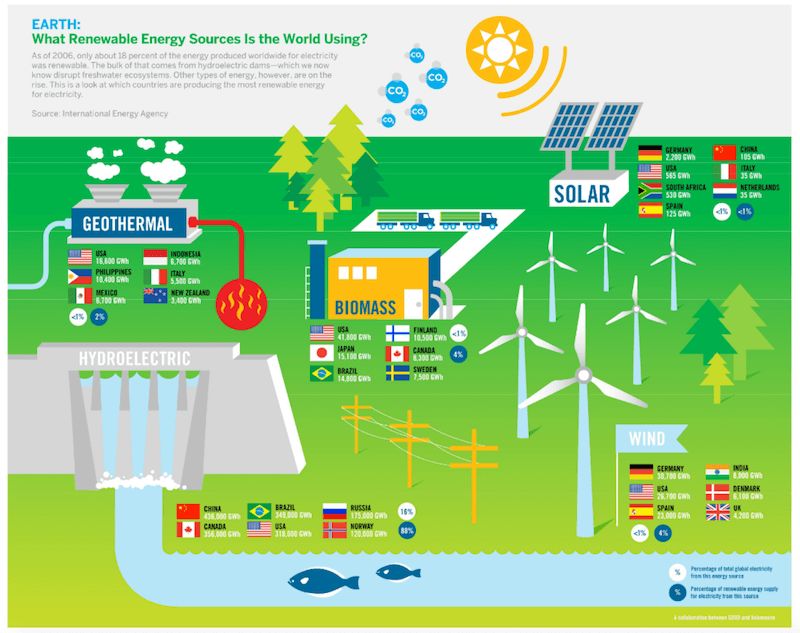
Renewable Energy
Renewable energy sources like solar, wind, hydroelectric, geothermal and biomass energy help conserve energy by capturing naturally occurring flows of energy and converting them into useful forms of power. For example:
Solar power harnesses energy from sunlight and converts it into electricity through the use of photovoltaic panels. This allows the radiant energy from the sun to be captured and utilized rather than being lost as heat.
Wind turbines convert the kinetic energy of wind into rotational mechanical power, which can then be converted into electricity. Harnessing the wind allows us to utilize this renewable flow of energy.
Hydropower utilizes the potential energy of water held at a height to generate electricity as it flows downwards through turbines. This converts the gravitational potential energy into useful electrical energy.
Geothermal power taps into the natural heat energy of the earth through geothermal heat pumps or steam from reservoirs of hot water. This geothermal energy can be harnessed to produce electricity.
Biomass power converts the chemical energy stored in organic plant matter into electricity through combustion or conversion into biofuels. The carbon absorbed by plants is recycled rather than being lost.
Overall, renewable sources allow us to conserve energy by capturing naturally occurring energy flows and converting them into useful forms of power in a sustainable way.
Energy Storage
While energy can never be perfectly conserved due to unavoidable losses, storing excess energy can help offset these losses and improve overall energy efficiency. There are several ways energy can be stored for later use:
Batteries – Electrochemical devices that convert chemical energy into electrical energy and vice versa. Batteries like lithium-ion are used to store energy from renewable sources like solar panels.
Flywheels – Mechanical devices that store rotational kinetic energy. As they spin, flywheels conserve energy for brief bursts of high power.
Pumped hydro – Stores energy by pumping water uphill into a reservoir, then releasing it through hydro turbines to generate electricity on demand.
Compressed air – Air is compressed and stored under pressure in underground caverns. The pressurized air can then turn turbines to produce electricity.
Hydrogen fuel cells – Use renewable electricity to split water into hydrogen and oxygen. The hydrogen can then be stored and used in fuel cells to generate electricity.
Energy storage allows renewable energy sources to offset times when energy demand exceeds supply. This reduces the need for fossil fuel power plants to handle peak loads.
Conclusion
In summary, the conservation of energy refers to the principle that energy can neither be created nor destroyed, but can only be transferred from one form to another. This is formalized in the First Law of Thermodynamics. When energy appears to be “lost” or dissipated, it is actually just being converted to a less usable form, such as waste heat. However, there are some real world inefficiencies in energy conversion processes, where some energy is genuinely lost to the environment. This loss can be minimized through technologies and designs that maximize energy efficiency. Ultimately, while energy can only be converted between different forms, some loss of usable energy does inevitably occur.
So in practical terms, energy conservation refers to the effort to reduce energy waste and maximize efficiency, even if total energy is not perfectly conserved. With wise use of renewable energy sources and improving technology, energy conservation allows us to make the most of our limited energy resources.