Is Natural Hydrogen Renewable?
Hydrogen is the most abundant chemical element in the universe. Here on Earth, hydrogen exists naturally in compounds like water and natural gas. When hydrogen is separated from these compounds, it emerges as a clean, high-energy fuel with a wide range of potential applications.
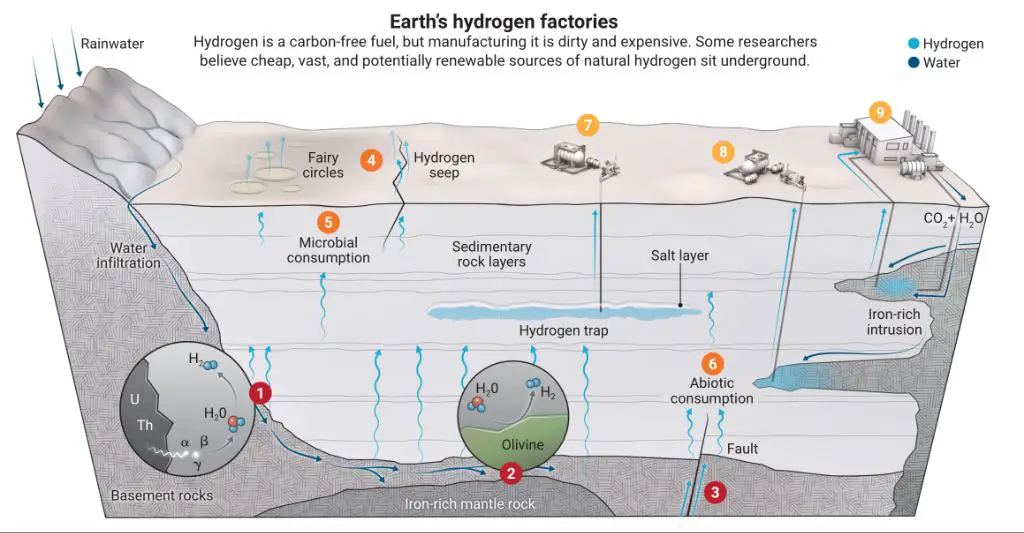
Natural hydrogen forms through geological processes in the Earth’s crust. It can slowly seep to the surface and be captured, most commonly from subsurface gas deposits and hot springs. Natural hydrogen production does not require human intervention or energy input.
This makes natural hydrogen distinct from artificially produced hydrogen. While artificial hydrogen is generated through methods like electrolysis that require electricity, natural hydrogen forms naturally and can be collected passively. Understanding the renewability and sustainability of natural hydrogen is important as we transition to cleaner energy sources.
Sources of Natural Hydrogen
Hydrogen occurs naturally on Earth through various chemical and geological processes. The main natural sources of hydrogen production are:
Chemical reactions – Hydrogen is produced when certain metals react with water or acids. For example, iron reacts with water vapor to produce iron oxide and hydrogen. Some bacteria also produce hydrogen through metabolic chemical reactions.
Biochemical processes – Certain types of algae and bacteria produce hydrogen through photosynthesis and fermentation. During these processes, enzymes help break down organic matter, releasing hydrogen as a byproduct.
Geothermal reactions – Hydrogen gas can be produced through reactions between water and the minerals found in hot rocks deep underground. This occurs near hydrothermal vents and volcanoes. The intense heat provides the energy needed to split water molecules into hydrogen and oxygen.
Some key references on natural hydrogen sources include:
https://www.eliis-geo.com/natural-hydrogen-the-new-eldorado-a.html
Renewability
Natural hydrogen is considered a renewable energy source because it is continuously produced by natural geological processes and is essentially inexhaustible (Wikipedia, 2023). The most common natural sources of hydrogen gas are reactions between water and rock occurring below the earth’s surface, at sites such as hydrothermal vents on mid-ocean ridges. At these high-temperature and high-pressure environments, water interacts with iron and magnesium-bearing rocks, releasing hydrogen gas as a byproduct. This geological hydrogen production occurs continuously and is estimated to refill consumed reserves on the scale of hundreds to thousands of years (USGS, 2023).
Unlike fossil fuels like oil, coal, and natural gas which take millions of years to form and are limited in supply, the natural production of hydrogen is an ongoing process that is not dependent on living biomass. As long as water continues flowing through hot rock reservoirs in the earth’s crust, new hydrogen gas will be generated (AGA, 2023). This makes natural hydrogen a renewable resource, as it is continually replenished by natural systems faster than it is consumed by human use.
Comparison to Fossil Fuels
Unlike fossil fuels like coal, oil and natural gas which are finite resources formed over millions of years, hydrogen can be produced continuously by natural processes. Fossil fuels exist in limited quantities and take an extremely long time to form naturally, so they are considered nonrenewable on human timescales. In contrast, natural hydrogen is generated through the ongoing processes of plants, algae and some microbes, making it a renewable energy source that can be produced indefinitely.
As the Marketplace article explains, fossil fuels have a clear cost advantage over hydrogen currently. However, as fossil fuel sources diminish over time, hydrogen produced through renewable processes could provide an alternative way to meet energy needs sustainably.
Environmental Impact
Compared to fossil fuels like coal, oil and natural gas, hydrogen offers significant environmental benefits when used as an energy source. When hydrogen is burned or used in a fuel cell, the only byproduct is water vapor, which means minimal air pollution is produced (1). Unlike fossil fuel combustion that emits greenhouse gases like carbon dioxide, burning pure hydrogen does not contribute directly to global warming or climate change.
According to MIT’s climate explainer on hydrogen Hydrogen, even if hydrogen is produced from natural gas today, total greenhouse gas emissions would be reduced by at least 20% compared to using the natural gas directly. And if renewable electricity like solar or wind is used to produce the hydrogen through electrolysis, greenhouse gas emissions can be cut by as much as 80% versus existing fossil fuel-based hydrogen production. This makes hydrogen a potentially promising way to decarbonize things like transportation and industry that have historically relied on high-emissions fuels like gasoline and diesel.
That said, hydrogen is not completely emissions free. Producing hydrogen from natural gas still creates some carbon emissions, and potential hydrogen leaks from storage and pipelines would also release greenhouse gases. But studies analyze the leakage rates and find overall climate benefits versus continued fossil fuel dependence, especially when hydrogen production shifts to lower-carbon sources. So while some consider hydrogen itself a greenhouse gas when leaked, the context shows its use as a fuel slashes emissions versus alternatives.
Challenges
There are several key challenges associated with utilizing natural hydrogen as an energy source. First, hydrogen sources like methane hydrate deposits and atmospheric hydrogen are diffuse and require advanced capture technologies (Source: https://100re-map.net/en/green-hydrogen-market-potentials-and-challenges/). Concentrating the hydrogen from these natural sources into a usable form is difficult given the current state of technology.
Second, existing energy infrastructure is not designed for hydrogen. Most pipelines, storage facilities, and power plants are built for natural gas, coal, or petroleum (Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10608639/). Converting these or building new infrastructure specifically for hydrogen’s unique properties represents a major challenge and cost. This infrastructure gap applies across the value chain from production to storage to end use.
Overall, utilizing natural hydrogen requires overcoming technical barriers in collection and concentration as well as enormous investments in dedicated infrastructure. Further research and development is needed to bring down costs and enable natural hydrogen to scale as an energy source.
Potential Applications
Natural hydrogen has several promising potential applications due to its high energy density and clean-burning properties when used as a fuel source. Key areas where natural hydrogen could be utilized include:
Fuel Cells – Hydrogen can be used to power fuel cells, which generate electricity through an electrochemical reaction. Fuel cells are highly efficient and produce water as the only byproduct. Natural hydrogen is well-suited for use in stationary fuel cells for buildings as well as fuel cells for electric vehicles.
Transportation – Hydrogen fuel cell vehicles are emerging as a zero-emission alternative to gasoline and diesel vehicles. Natural hydrogen could provide a renewable and abundant fuel source to power fuel cell vehicles such as passenger cars, trucks, buses, trains, ships, and airplanes.
Power Generation – Hydrogen can be burned directly in modified gas turbines to generate electricity. Power plants utilizing natural hydrogen would have zero emissions. Hydrogen may also enable greater use of intermittent renewable energy sources like wind and solar by storing excess energy production as hydrogen.
Future Outlook
The future role of natural hydrogen in the renewable energy transition is uncertain but promising. Some projections indicate that natural hydrogen could provide up to 10% of global energy demand by 2050 (hydrogenfuelledcars.com). Advances in extraction technologies and dropping costs could make natural hydrogen more viable and widespread. However, challenges around transportation, storage, and infrastructure will need to be addressed. Overall, natural hydrogen has potential to be part of the renewable energy mix but its growth depends on technical and economic developments.
Comparisons to Artificial Hydrogen
While natural hydrogen is produced from biological or geological processes, artificial hydrogen is synthesized through industrial processes like electrolysis or steam methane reforming. These artificial methods require significant energy inputs, often derived from fossil fuels, making artificial hydrogen more expensive and carbon intensive to produce.
Electrolysis uses electricity to split water molecules into hydrogen and oxygen. The source of this electricity plays a key role in determining the sustainability of the resulting hydrogen. Renewable electrolysis using solar, wind, or hydropower can produce low-carbon hydrogen, but fossil fuel-based electricity makes it a high emission process. Steam methane reforming is also carbon intensive as it converts natural gas into hydrogen and carbon dioxide.
According to the U.S. Department of Energy, the current cost of producing a kilogram of hydrogen through electrolysis ranges from $5-7, while steam methane reforming costs around $1-2/kg. In contrast, natural hydrogen produced through biogas reformation or gasification can be as low as $1-3/kg. While renewable electrolysis costs are expected to fall with more solar/wind deployment, natural hydrogen is likely to maintain a considerable cost advantage.
Overall, natural hydrogen leverages existing biological and geological cycles to renewably produce hydrogen at a lower financial and environmental cost compared to current artificial production methods. As artificial techniques like electrolysis continue improving in efficiency and sustainability, natural hydrogen will remain an abundant, low-cost, and renewable alternative.
Conclusion
To summarize, hydrogen is an abundant element that can be renewable if produced through renewable resources like solar, wind, or hydro power. While hydrogen itself is not inherently renewable, the methods and energy sources used to produce it determine whether it is considered a renewable fuel. When compared to fossil fuels, hydrogen produced from renewables has clear environmental benefits through reduced emissions. However, there are still challenges around efficiency, storage, transportation and cost that need to be addressed before hydrogen can be widely adopted as an energy source. The future applications and scalability of renewable hydrogen remain promising, but will require further development of production, infrastructure, and innovative technologies. Ultimately, renewable hydrogen has the potential to play a key role in a more sustainable energy future, but its renewability depends on how it is produced.