Is Light Energy Yes Or No?
Define Light Energy
Light energy is a form of electromagnetic radiation. It is made up of particles called photons that travel in waves. The wavelength and frequency of the photons determine the properties and behavior of the light.
Visible light that humans can see is only a small part of the full electromagnetic spectrum. The wavelengths of visible light range from around 380 to 750 nanometers. Other forms of electromagnetic radiation like radio waves, microwaves, infrared radiation, ultraviolet rays, X-rays and gamma rays have longer or shorter wavelengths than visible light.
Photons carry energy based on their wavelength and frequency. Shorter wavelength, higher frequency photons like gamma rays or X-rays carry the most energy. Longer wavelength, lower frequency photons like radio waves carry the least energy. The energy carried by photons is directly proportional to their frequency and inversely proportional to their wavelength.
Properties of Light
Light exhibits some unique properties that distinguish it from other forms of energy or radiation. Some key properties of light include:
Wavelength and Frequency
Light behaves as a wave, oscillating up and down at particular wavelengths and frequencies. The wavelength of light determines its color – shorter wavelengths are blue/violet while longer wavelengths are red. The frequency refers to the number of wave oscillations per second.
Speed
Light travels extremely fast – approximately 186,000 miles per second. This speed is constant in a vacuum, and forms the cosmic speed limit.
Dual Nature
Light exhibits properties of both waves and particles. The wave properties were traditionally studied, but early 20th century quantum mechanics revealed light’s particle nature as packets of energy called photons.
Overall, the wavelength, frequency, speed, and dual wave-particle nature make light distinct from other entities. These special properties lead to many of light’s applications across science and technology.
Light as a Form of Energy
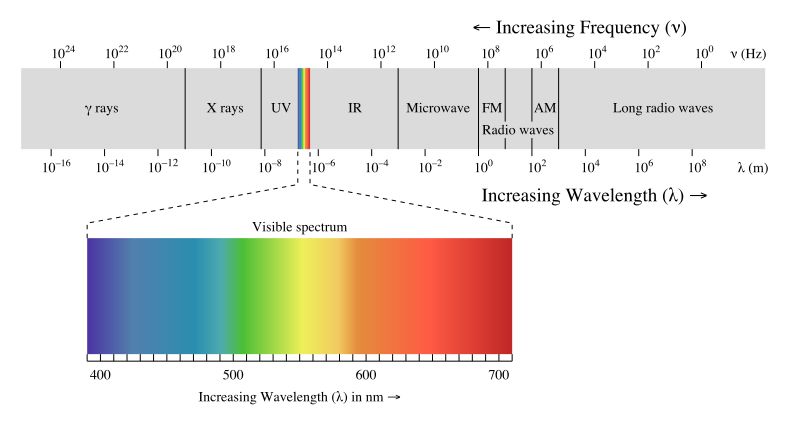
Light is considered a form of energy for several key reasons. Most importantly, it falls within the electromagnetic spectrum, which encompasses all the different frequencies and wavelengths of electromagnetic radiation. The electromagnetic spectrum includes, from lowest to highest frequency, radio waves, microwaves, infrared light, visible light, ultraviolet light, X-rays and gamma rays. These are all fundamentally forms of light energy, just at varying frequencies.
Visible light that humans can detect with their eyes is just one small slice of the full electromagnetic spectrum. But all forms of electromagnetic radiation can be described in terms of photons, discrete packets of light energy. The energy of a photon depends on its frequency, according to Planck’s equation. Higher frequency photons like X-rays pack a lot more energy than lower frequency radio waves.
A key characteristic of energy is its capacity to do work. Light energy can exert pressure and do mechanical work. We see this with solar sails in space, where sunlight provides propulsive force to move spacecraft. On a more everyday scale, light energy from the Sun evaporates water and fuels photosynthesis in plants and other chemical reactions. Light can also carry momentum and exert pressure on objects, an effect Einstein helped demonstrate with his work on the photoelectric effect.
So in summary, because light behaves like waves, transports energy according to its frequency, and can do work, it meets the criteria for being a form of energy.
Evidence Supporting Light as Energy
There are several key pieces of evidence from science that demonstrate light behaves as a form of energy:
The photoelectric effect shows that light interacting with matter can cause electrons to be emitted, which is a form of energy transfer. When light shines on certain metals, electrons are ejected from the metal’s surface. The energy of the light is absorbed by the electrons, allowing them to overcome the metal’s binding force and be released as kinetic energy. The photoelectric effect was pivotal in demonstrating the particle properties of light.
Solar panels and solar cells convert light directly into electrical energy through the photovoltaic effect. Photons from sunlight hit the solar cell material (usually silicon), knocking electrons loose. This generates an electric current that can then be harnessed for power. The operation of photovoltaics provides direct evidence of light’s energy.
Counter Arguments
While light is widely accepted as a form of energy by the scientific community, there are some counter-arguments and common misconceptions around this topic:
Some argue that since light has no mass, it cannot be a form of energy. However, mass is not a requirement for something to contain energy. Kinetic energy, thermal energy, and electromagnetic radiation like light all qualify as energy without possessing mass.
Others contend that light only transfers energy, but does not comprise energy itself. Yet the very ability of light to transfer energy indicates that it must contain energy. The laws of thermodynamics state that energy cannot be created or destroyed, only converted from one form to another. Light can convert into other verifiable forms of energy, proving it too possesses energy.
A common misconception is that light does not meet the definition of “work” in physics, since it exerts no force over a distance. However, moving an object is not the sole qualification for doing work. Light does produce physical changes in matter through electrochemical reactions, fluorescence, photosynthesis and more, indicating useful energy output.
While counter-arguments may provide thought-provoking discussion, the overwhelming scientific evidence supports classifying light as a fundamental form of energy. The debates illuminate our evolving understanding, but do not negate the experimental, mathematical and practical proof of light’s energetic nature.
Laws of Thermodynamics
Light relates to the laws of thermodynamics in a few key ways.
The first law of thermodynamics states that energy can neither be created nor destroyed, only transformed from one form to another. When light photons interact with matter, the photons can be absorbed, raising the energy state of electrons within the matter. This demonstrates the transformation of light energy into other forms. The absorption of light energy complies with the conservation of energy required by the first law.
The second law of thermodynamics states that the entropy of an isolated system always increases over time. When light is emitted from a source and absorbed by matter, the photons tend to scatter and spread out. This increase in disorder aligns with the second law. Additionally, when light raises electrons to excited states, they will release energy as they relax back to ground states. This process increases entropy.
Overall, the behaviors and interactions of light follow and confirm both laws of thermodynamics. The conversion of light to other energy forms upholds the first law, while the dispersal and degradation of light over time complies with the second law’s mandate of ever-increasing entropy.
Everyday Examples of Light as Energy
There are many everyday examples that demonstrate light’s role as a form of energy:
Photosynthesis
Plants absorb light energy from the sun through photosynthesis. This light energy is converted into chemical energy that the plant uses to grow and thrive.
Vision
Our eyes contain light receptors that convert light energy into electrical signals. These signals are sent to our brain, allowing us to see.
Sunburn
Overexposure to sunlight can result in sunburn. This is because the high-energy ultraviolet light damages skin cells.
These common experiences clearly show that light transmits energy in the form of radiation that can be captured, converted, and sometimes cause damage.
Technological Applications
Light energy has enabled numerous technologies that we rely on today. Some notable examples include:
Solar Cells – Also known as photovoltaic cells, solar cells convert sunlight directly into electricity. They are made of semiconducting materials that absorb photons from sunlight and release electrons, generating an electric current. Solar cells have enabled clean renewable energy from the sun.
Lasers – Lasers amplify light through stimulated emission of electromagnetic radiation. The term “laser” stands for Light Amplification by Stimulated Emission of Radiation. Lasers have applications in manufacturing, medicine, military, and more.
Fiber Optics – Fiber optic cables transmit data over long distances by enclosing streams of light inside thin glass strands. The light signals can carry telephone, internet, and television signals. Fiber optics have enabled high-speed long distance communication networks.
Historical Understanding
Humans have pondered the nature of light and studied its properties for thousands of years. Some key discoveries relating to light as a form of energy include:
350 BCE – Greek philosophers like Aristotle describe light as the “activity of what is transparent”, hypothesizing that light required a medium like air or water to travel through. This begins scientific inquiry into the nature of light.
1665 CE – Isaac Newton uses a prism to demonstrate that sunlight is made up of a spectrum of colors, laying the groundwork for understanding wavelengths of light.
1800 – English chemist William Herschel discovers infrared radiation through experiments with thermometers and prisms, showing light contains unseen rays with heating effects.
1864 – Scottish physicist James Clerk Maxwell publishes equations unifying electricity, magnetism and light as electromagnetic waves traveling at the speed of light.
1900 – German physicist Max Planck introduces the quantum hypothesis that energy is emitted and absorbed in discrete quanta, starting quantum mechanics. This explained blackbody radiation of different wavelengths.
1905 – Albert Einstein proposes the photoelectric effect, whereby photons striking a metal surface cause emission of electrons. This demonstrated light has particle-like properties.
1917 – Einstein establishes the corpuscular theory of light, showing light has both wave and particle properties. This growing understanding cemented light’s nature as a form of energy.
Conclusion
Light qualifies as a form of energy based on several key pieces of evidence. First, light exhibits behaviors consistent with other forms of energy, like the ability to do work and transfer between different systems. Additionally, light follows the established laws of thermodynamics that govern energy transactions and transformations. On a practical level, we can observe light’s energetic properties in real world applications like solar panels, photosynthesis, laser cutting, and more. Historically, our understanding of light as energy evolved over centuries of scientific discovery, beginning with early observations by thinkers like Newton and Huygens and confirmed definitively with Maxwell’s electromagnetic theory in the 19th century. While fringe viewpoints persist, the preponderance of scientific evidence firmly establishes light as a bona fide form of energy, interchangeable with other forms like heat, motion, electricity and more.
In summary, light unambiguously meets the criteria to qualify as a form of energy based on its behavior, conformity to thermodynamic laws, practical applications converting light to other energy forms, and the overall scientific consensus from decades of accumulated knowledge.