Is Light Energy Potential Or Kinetic Energy?
Light energy is a form of electromagnetic radiation that can be detected by the human eye. It consists of photons, or discrete packets of energy, that travel in waves. There has been extensive debate among physicists over whether light should be considered a form of potential energy or kinetic energy. While light exhibits wavelike properties that suggest potential energy, it also demonstrates particle-like properties that imply kinetic energy. This results in the unique dual nature of light, which makes a definitive classification challenging.
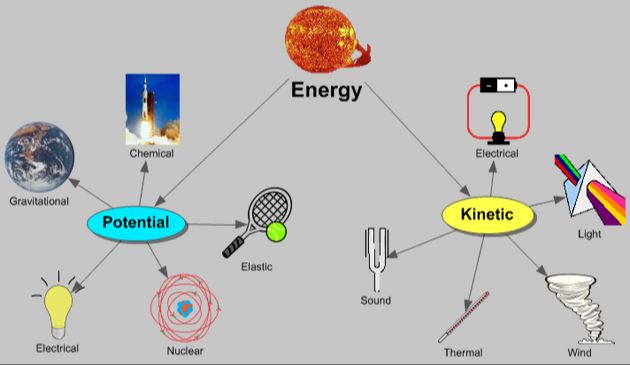
Definitions of Potential and Kinetic Energy
Potential energy is stored energy that has the potential to do work. It is energy possessed by an object by virtue of its position or state. For example, energy stored in a spring or water held behind a dam are forms of potential energy. The energy is only released and converted into kinetic energy when the object is allowed to change its state – the spring decompresses or the water flows. Other common examples are objects held at a height due to gravity, like a rock on a cliff or water in a reservoir.
Kinetic energy is the energy of motion. It is the work needed to accelerate an object to a certain speed. The faster the object moves, the more kinetic energy it possesses. Common examples of kinetic energy are a rolling ball, flowing water, or the motion of wind. Kinetic energy can be transferred between objects. For instance, when one billiard ball strikes another, it transfers some of its kinetic energy to the second ball, causing it to move.
Properties of Light
Light is a form of electromagnetic radiation, which means it is a type of energy that travels as waves and behaves like both a wave and a particle. Light has specific properties that define how it interacts with matter.
As a wave, light has a wavelength and frequency. Wavelength refers to the distance between successive wave crests, while frequency refers to the number of wave cycles that pass a fixed point per unit of time. The wavelength and frequency of light are related – the shorter the wavelength, the higher the frequency.
Visible light that humans can see ranges in wavelength from about 380 to 700 nanometers (nm). Other forms of electromagnetic radiation like radio waves, microwaves, X-rays and gamma-rays have different wavelengths and frequencies than visible light. Frequency is measured in hertz (Hz), or cycles per second.
Another key property of light is speed. In a vacuum like outer space, light travels at an extremely fast speed of approximately 186,000 miles per second (300,000 kilometers per second). This speed is known as the speed of light and is considered a fundamental constant of nature.
Understanding the dual wave-particle nature of light along with its wavelength, frequency and speed are foundational to explaining many phenomena involving light in physics.
Light as a Wave
Light can be modeled as a wave due to its oscillating electric and magnetic fields which create electromagnetic waves. These waves have oscillating frequencies and amplitudes but no mass. The oscillations of light waves demonstrate their inherent motion and kinetic energy, much like how ocean waves move with kinetic energy. As the electromagnetic light waves propagate through space, the oscillating electric and magnetic fields store and release energy rhythmically. This motion of the light wave’s energy is directly related to kinetic energy.
According to the kinetic theory of matter, kinetic energy arises from the motion of objects. Since light waves exhibit oscillatory motion as they travel, they can be considered to have kinetic energy. The kinetic energy contained within a light wave depends on its intensity and frequency – higher intensity and frequency correspond to greater kinetic energy. Even though individual photons (particles of light) have no mass, they still move at the speed of light and thus possess kinetic energy equal to their total energy. Overall, modeling light as a moving wave illustrates how it inherently contains kinetic energy due to its oscillatory motion.
Light as a Particle
According to the photon model, light can be modeled as discrete packets or quanta of energy called photons [1]. Photons carry energy proportional to the frequency of the associated electromagnetic wave according to the equation E=hf, where E is the energy of the photon, h is Planck’s constant, and f is the frequency [2]. This quantized nature of light energy was proposed by Einstein in 1905 to explain the photoelectric effect, in which shining light onto a metal surface causes the emission of electrons. The energy of the emitted electrons depends only on the frequency, not the amplitude, of the incident light.
Since the energy is quantized into photons, the energy can be viewed as potential energy carried by the photons. The photon energy E=hf represents the potential energy of each indivisible quantum of light. When a photon is absorbed by matter, this potential energy can be transferred and induce effects like the photoelectric effect. In this way, the photon model relates the observed discrete behavior of light energy to potential energy stored in individual photons [3].
Dual Nature of Light
Light exhibits properties of both waves and particles, a concept known as wave-particle duality. According to wave-particle duality, quantum mechanical entities can behave as waves or particles depending on the experimental setup or measurement made (Wikipedia, 2023). This means that light can demonstrate characteristics of waves, such as interference and diffraction, as well as particle-like behavior like the photoelectric effect. The wave-particle duality of light was first proposed by Louis de Broglie in 1924, who suggested that not only light but all matter exhibits wave-particle duality.
Evidence for the dual nature of light comes from both the wave and particle perspectives. As a wave, light can be diffracted and interfered with itself. But light also demonstrates particle properties – it comes in discrete quanta called photons that carry momentum and can be absorbed or emitted. Wave-particle duality arises from the complex mathematics of quantum mechanics, in which the equations predict both wave-like and particle-like behavior. Thus light is neither exclusively a wave nor exclusively a particle, but possesses characteristics of both depending on the type of experiment or measurement performed.
The discovery of wave-particle duality revolutionized physics in the early 20th century and was a key step in the development of quantum mechanics. It showed that the classical concepts of waves and particles were incomplete, and that a new probabilistic interpretation was needed at the quantum scale. Wave-particle duality is central to our understanding of the behavior of photons, electrons, and all quantum entities.
Evidence for Light as Kinetic Energy
There are several key experiments that demonstrate light exhibits wave-like properties, supporting the idea that light is a form of kinetic energy. The photoelectric effect, discovered by Heinrich Hertz in 1887, showed that light shining on a metal surface causes electrons to be emitted from the surface [1]. The kinetic energy of the emitted electrons depends on the frequency/amplitude of the light, meaning the light must transfer kinetic energy to the electrons to eject them from the metal. This provided direct evidence of light’s kinetic nature.
Additionally, light exerts radiation pressure on objects, the ability to transfer momentum and impart motion. Crookes radiometer, invented in 1873, uses this principle to spin vanes in a partial vacuum when exposed to light. Since kinetic energy is defined as the energy possessed by an object due to its motion, the ability of light to impart motion and momentum demonstrates it carries kinetic energy [2].
Evidence for Light as Potential Energy
When looking at light as discrete particles or photons, there is evidence that light exhibits potential energy. According to the photon model, light energy is quantized into indivisible packets or photons [1]. Photons contain energy but have no mass. This energy is considered potential energy since it is stored in the photon and can be absorbed or emitted during atomic transitions [2].
When an electron in an atom absorbs a photon, it gains the energy contained within the photon and moves to a higher energy state. This absorbed energy is now potential energy stored in the excited electron. Likewise, when an excited electron decays to a lower energy state, it emits a photon containing energy equal to the energy difference between the two states. Thus the potential energy stored in the excited electron is released as a photon. This demonstrates the potential energy nature of light according to the photon model [1].
Conclusion
There has been an ongoing debate regarding whether light energy is kinetic or potential energy. Some key evidence shows that light exhibits properties of kinetic energy – it is always in motion as electromagnetic waves and consists of massless particles called photons that are moving. Yet, other evidence indicates light can also be considered a form of potential energy – it can exist in a bound state and be released like the potential energy stored in chemical bonds. Light’s dual wave-particle nature is complex.
In conclusion, the question of whether light is kinetic or potential energy leads to the insight that light has characteristics of both kinetic and potential energy. As waves, light propagates through space and must have kinetic energy to travel. But light can also be absorbed and emitted between atoms in discrete amounts like potential energy. Overall, light fundamentally exhibits the properties of both kinetic and potential energy simultaneously.
References
[1] Einstein, Albert (1905). “On a Heuristic Viewpoint Concerning the Production and Transformation of Light”. Annalen der Physik. 17 (6): 132–148. Bibcode:1905AnP…322..132E. doi:10.1002/andp.19053220607.
[2] Newton, Isaac (1704). Opticks: Or, A Treatise of the Reflections, Refractions, Inflections and Colours of Light. London: Sam. Smith & Benj. Walford.
[3] Maxwell, James Clerk (1865). “A Dynamical Theory of the Electromagnetic Field”. Philosophical Transactions of the Royal Society of London. 155: 459–512. doi:10.1098/rstl.1865.0008.
[4] Planck, Max (1901). “On the Law of Distribution of Energy in the Normal Spectrum”. Annalen der Physik. 4 (3): 553 ff. Bibcode:1901AnP…309..553P. doi:10.1002/andp.19013090310.