Is It True That Energy Cannot Be Created Or Destroyed?
The law of conservation of energy is one of the most fundamental laws in all of physics. This law states that energy can neither be created nor destroyed – it can only be transformed from one form to another. This principle forms the very basis of thermodynamics and has implications in all areas of science. The general concept behind this law is that there exists a quantity of energy in a closed system which remains constant, though it may change forms.
History
The law of conservation of energy was first proposed in the 1840s by German physician and physicist Julius Robert von Mayer. Mayer was studying human metabolism and physiology when he came to the realization that energy can be transformed from one form to another but can never be created or destroyed. This principle came to be known as the first law of thermodynamics.
Mayer’s work built upon previous contributions in physics and engineering, including concepts like latent heat and vis viva from scientists such as James Joule. However, Mayer was the first to propose a universal law of conservation of energy. His papers published between 1841-1848 outlined this concept and its applications to areas like physiology, meteorology, and mechanics.
While Mayer’s proposals were initially met with skepticism, the law of conservation of energy gained wider acceptance in the 1850s after similar conclusions were reached by scientists such as Hermann von Helmholtz in Germany and William Thomson (Lord Kelvin) in Britain. Over time, the law of conservation of energy became a fundamental principle of physics that underlies many scientific disciplines.
Explanation
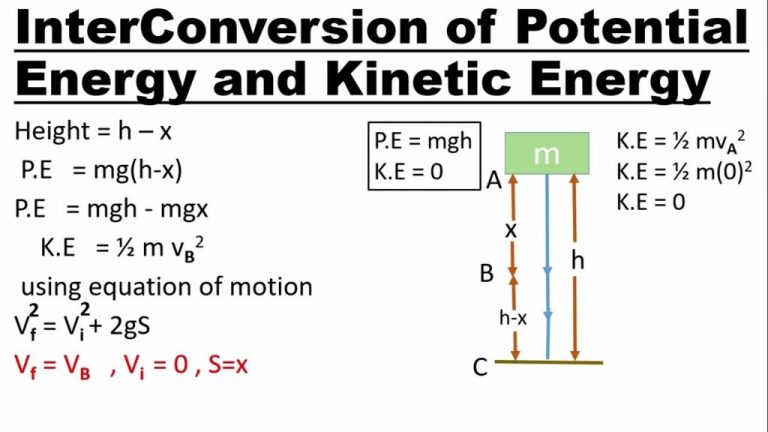
Energy can be defined as the capacity to do work. There are different forms of energy such as potential energy, kinetic energy, thermal energy, electrical energy, chemical energy, nuclear energy, and more. Potential energy is the stored energy an object has due to its position or state. For example, a ball held at a height above the ground has potential energy due to gravity. Kinetic energy is the energy of motion that a moving object possesses. The faster an object moves, the more kinetic energy it has.
The law of conservation of energy states that energy cannot be created or destroyed, but only transformed from one form into another. For example, when the ball is dropped, its potential energy is transformed into kinetic energy as it falls. When it hits the ground, the kinetic energy is transformed into heat and sound. While the forms of energy change, the total amount of energy in the closed system remains constant.
Applications
The law of conservation of energy has many real-world applications across science and technology. Here are some examples:
Mechanical Engineering: Engineers apply the law of conservation of energy when designing machines and systems to maximize efficiency. The law enables them to track energy transfers and transformations, like converting fuel into kinetic energy to power vehicles.
Thermodynamics: The laws of thermodynamics rely heavily on the conservation of energy. Thermodynamic processes like heating/cooling, compressing gases, or generating electricity all obey the fundamental rule that energy must be conserved.
Biology: Living organisms require energy to grow, move, and function. The conservation of energy principle governs how they utilize chemical energy from food to power biological processes. It explains how energy flows through ecosystems.
Chemistry: Chemical reactions involve rearranging atoms, which cannot create or destroy energy. Bond breaking consumes energy and bond forming releases energy, but the total energy remains constant.
Nuclear Physics: Nuclear processes like fission and fusion obey the conservation of energy rule. The combined rest mass and binding energies of particles before a reaction equal the total mass-energy afterwards.
Overall, the law provides a foundation for making accurate calculations and predictions across the natural sciences and engineering fields.
Limits
While the law of conservation of energy is considered a fundamental principle in physics, there are certain situations where it may seem not to apply.
One example is in quantum mechanics, where temporary violations of conservation of energy at the quantum level appear possible due to the uncertainty principle. Virtual particles can briefly come into existence and vanish, with energy conserved only on average over time.
Another potential exception is in systems with unusually high gravitational fields, such as near the event horizon of a black hole. Here, quantum effects and general relativity make the law of conservation of energy difficult to apply.
Additionally, some cosmological models suggest that conservation of energy did not hold in the very early universe shortly after the Big Bang. The expansion and evolution of space-time may have violated the conservation law initially, only settling into its present stable form over time.
However, most physicists believe these are technical quibbles, and that the conservation law truly reflects an underlying symmetry of nature. The exceptions only occur under extreme conditions rarely seen in everyday phenomena. For all practical purposes, energy remains conserved in closed systems under normal circumstances.
Misconceptions
Despite the fundamental nature of the law of conservation of energy, there are some common misconceptions surrounding it:
Perpetual motion machines – The idea of a perpetual motion machine that can generate useful energy indefinitely without an external power source contradicts the law of conservation of energy. Such machines are impossible in practice because of inevitable energy losses to friction and other inefficiencies.
Creating energy – It’s commonly believed that energy can be “created” by humans, when in fact it can only be converted from one form to another. For example, we don’t create electrical energy but rather convert chemical energy from fuels into electricity.
Destroying energy – Some think energy can be destroyed over time, but it can only be transformed or transferred. The total amount of energy in an isolated system always remains constant.
Generating energy from nothing – Devices that claim to create significant amounts of energy from vacuum, zero-point energy, or cosmic rays violate the law of conservation of energy. There is no known way to produce useful energy from nothing.
The only exception to the principle of conservation of energy is when mass gets converted to energy, as in nuclear reactions. But even then, the total quantity of mass-energy remains unchanged.
Counterarguments
While the law of conservation of energy is considered a fundamental principle of physics, there have been some challenges and objections to its universality over the years:
– Some have argued that the law may break down at the quantum level or in extreme gravitational fields like black holes. Virtual particle pairs can spontaneously appear and annihilate, which seems to violate conservation of energy on very small scales.
– Others contend conservation of energy doesn’t apply to an expanding universe, where new space and energy can be created. The total energy of the universe may not remain constant as it expands.
– A few theories in cosmology like steady state theory posit mechanisms for continuously creating new matter to maintain a static universe. This violates conservation of energy in order to avoid a universal heat death.
– Some interpretations of general relativity allow for anomalies like wormholes or warp drives that could permit faster-than-light travel, which might enable energy production from nothing.
– At the start of the universe during the Planck epoch, quantum effects dominated and conservation laws may not have fully applied yet. The initial inflationary expansion may not have conserved energy.
– On the theological side, some argue miracles or divine interventions allow for temporary energy creation and thus violate strict conservation.
However, most of these objections have scientific counter-arguments or remain speculative. The universality of energy conservation is supported by extensive experimental verification and remains a foundational part of modern physics.
Supporting Evidence
There is considerable experimental support for the law of conservation of energy. Scientifically controlled experiments have consistently shown that when all forms of energy are accounted for, the total energy before and after a process remains unchanged. Here are some examples:
- Chemical reactions: Chemists track the energy content of reactants and products. Exothermic reactions like combustion convert chemical energy into heat, while endothermic reactions require heat input, but in both cases energy is conserved.
- Nuclear processes: Careful measurements during nuclear reactions like fission, fusion, and radioactive decay show consistent energy balances.
- Phase changes: Heating or cooling matter so it changes state (e.g. ice to water) requires energy input or output, but the system energy remains fixed.
- Mechanical systems: The kinetic and potential energy changes in pendulums, springs, and mechanical collisions always sum to a constant amount.
- Thermal systems: Energy audits of home insulation, engines, refrigerators, and other systems reveal quantitative compliance with energy conservation.
Across every domain of science, experiments uphold the universal law of conservation of energy. While energy can change form, quantitative measurements always show it is neither created nor destroyed.
Recent Developments
While the law of conservation of energy has remained constant, scientists continue to gain new insights into how energy transforms within systems. Some recent developments include:
– Advances in thermodynamics have provided a deeper understanding of how heat, work, and internal energy are interrelated in both classical and quantum systems.
– New technologies like solar cells allow us to more efficiently convert certain forms of energy like light into electricity for human use.
– Discoveries in particle physics reveal new subatomic particles that may play roles in energy transformations that we don’t yet fully comprehend.
– Astrophysicists now believe that dark energy, an unknown form of energy, may be causing the expansion of the universe to accelerate.
– Quantum entanglement suggests energy transformations may occur non-locally, challenging previous assumptions.
While the total amount of energy remains constant, these discoveries reveal new facets of how energy transforms and highlight that there are still unsolved mysteries in our understanding of energy.
Conclusion
In summary, the law of conservation of energy is one of the most fundamental laws in physics. It states that the total energy in an isolated system always remains constant – it cannot be created or destroyed, only converted from one form to another.
This law has important implications in science. It means that perpetual motion machines are impossible, as no system can produce an unlimited amount of energy. The law also enables us to track the flow of energy through systems and account for energy gains and losses during processes.
The conservation of energy is crucial to our understanding of the universe. It allows us to make predictions and models energy transactions with high accuracy. The law is considered to be held as an absolute truth, with no valid exceptions ever observed. It will continue to be a keystone of physics theories going forward.
While new forms of energy are discovered and new technologies developed, the total energy of any closed system remains fixed. The law of conservation of energy will likely stand as one of the most fundamentally important natural laws for all future scientific inquiries.