Is Hydrogen A Possible Energy Source?
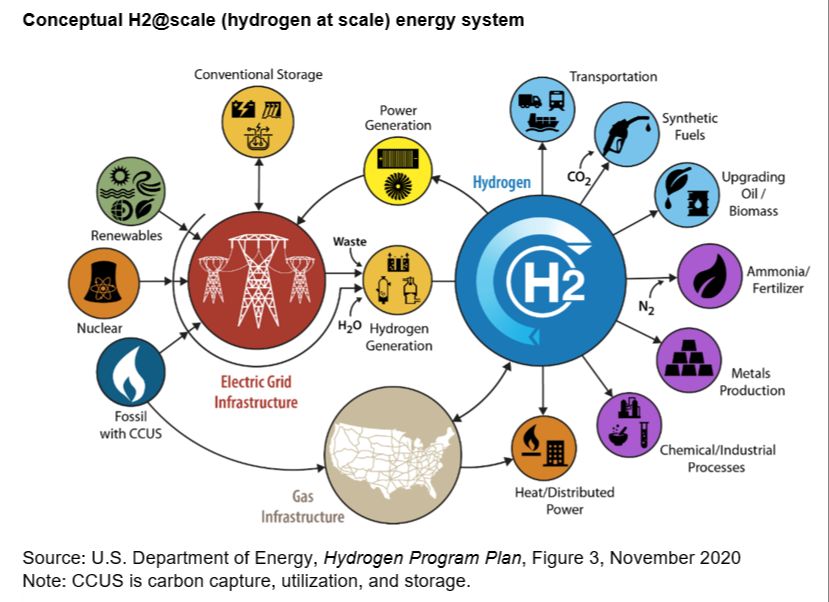
Hydrogen is the most abundant chemical substance in the universe. It’s an odorless, colorless, and highly combustible gas. When used as an energy source, hydrogen gas is converted to electricity through fuel cells or burned with oxygen to create heat. The only byproduct from these reactions is water, making hydrogen a clean fuel source. Hydrogen energy is being explored as an alternative to fossil fuels because it does not produce greenhouse gas emissions like carbon dioxide when used. With concerns about climate change and energy security, hydrogen is attractive because it can be produced domestically from resources like natural gas, nuclear power, biomass, and renewable power like solar and wind. Hydrogen has the potential to provide energy for transportation, electricity generation, and industrial sectors. This overview will examine hydrogen as an energy source, including production methods, uses, environmental impact, economic viability, storage and transportation challenges, safety considerations, and the future outlook.
Hydrogen Production Methods
There are several ways that hydrogen can be commercially produced on a large scale. Some of the main methods include:
Electrolysis: This process uses electricity to split water into hydrogen and oxygen. The reaction takes place in an electrolyzer. Electrolyzers can range from small on-site hydrogen generators to large dedicated hydrogen production plants. According to the US Department of Energy, low-temperature electrolysis and photoelectrochemical water-splitting are promising electrolysis technologies that are still in early research and development phases [1].
Natural Gas Reforming: The most common method is called steam methane reforming (SMR). This involves reacting methane from natural gas with high-temperature steam to produce hydrogen along with carbon monoxide and a small amount of carbon dioxide. SMR accounts for nearly all of the hydrogen produced in the United States today [2].
Biomass Gasification: Renewable hydrogen can also be produced from biomass via thermochemical processes like gasification. Here biomass Feedstocks like forest residues and agricultural crops are heated with a controlled amount of oxygen to create a synthesis gas containing hydrogen along with carbon monoxide and carbon dioxide [3].
Usage in Transportation
Hydrogen can be used to power fuel cell vehicles, which convert hydrogen and oxygen into electricity to run an electric motor. Fuel cell vehicles, like the Toyota Mirai and Honda Clarity Fuel Cell, emit only water vapor from their tailpipes and have ranges over 300 miles per fill up. Several major automakers, including Toyota, Honda, Hyundai, and Mercedes-Benz, currently offer fuel cell vehicle models.
A key challenge to more widespread adoption of fuel cell vehicles is the lack of hydrogen fueling stations. According to the U.S. Department of Energy, there are currently only 48 hydrogen fueling stations open to the public in the U.S., though many more are planned. California and several East Coast states have been expanding the number of stations, aiming to build the infrastructure needed to support consumer fuel cell vehicle adoption. More stations will be required for fuel cell vehicles to achieve mass-market success.
Usage in Electricity Generation
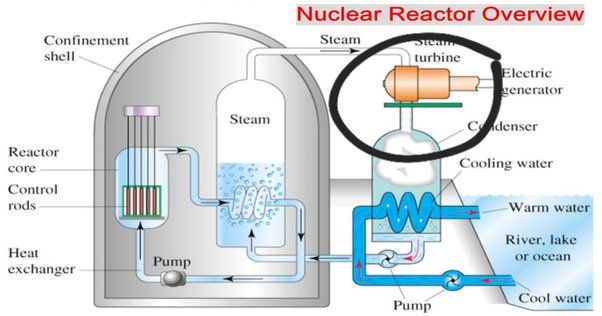
Hydrogen has emerged as a promising option for generating electricity with low carbon emissions. There are two primary ways hydrogen is used for electricity production: combustion in turbines and fuel cells.
Hydrogen can be burned directly in gas turbines, similar to natural gas, to spin a generator and produce electricity. The only byproducts from hydrogen combustion in turbines are water and small amounts of nitrogen oxides. This makes hydrogen a clean fuel source for electricity generation. One challenge with hydrogen turbines is that burning pure hydrogen can produce high flame temperatures that require modifying turbine materials. However, mixing hydrogen with natural gas allows it to be used in conventional gas turbines. Studies have found hydrogen-natural gas blends up to 30% hydrogen by volume can be used in existing turbines with minimal changes.
Fuel cells provide another carbon-free pathway for extracting electricity from hydrogen. Fuel cells generate electricity through an electrochemical reaction, not combustion, combining pure hydrogen and oxygen. This reaction produces only water and heat as byproducts. Fuel cell systems can achieve efficiencies over 60% in electricity production, higher than traditional thermal power plants. While more expensive currently, fuel cells are modular and able to provide resilient backup power. With continued cost reductions, hydrogen fuel cells may become a major technology for clean electricity production.
Environmental Impact
Hydrogen itself is a clean burning fuel that only produces water vapor when used. However, the methods of producing hydrogen can have varying environmental impacts. Most hydrogen today is produced from natural gas in a process called steam methane reforming. This process generates significant carbon emissions. Some methods like electrolysis using renewable electricity can produce very low emission “green” hydrogen. But electrolysis is currently more expensive than fossil fuel-based production (source).
According to an analysis by Energy Innovation, roughly 30% of hydrogen projects in the U.S. use fossil fuels as their energy source. They estimate that green hydrogen currently makes up less than 1% of total global hydrogen production (source). More work needs to be done to scale up green hydrogen production in order for hydrogen to be considered a clean fuel across its full life cycle.
Economic Viability
Hydrogen production and infrastructure currently comes at a high cost compared to conventional fossil fuels. According to the International Energy Agency (IEA), the average cost of producing hydrogen from renewable electricity (green hydrogen) was around $5/kg in 2019, while fossil-based hydrogen (grey/blue) averaged around $1.50/kg (CNBC). However, costs are expected to decline as technology improves and scale increases. The IEA projects renewable hydrogen costs could fall to $1-2/kg by 2050.
In the near term, government subsidies will be important to make hydrogen cost-competitive. Many governments globally have set hydrogen production and infrastructure investment targets and are offering tax credits, loans and other incentives. For example, the U.S. Department of Energy announced in 2021 it will invest over $8 billion in hydrogen hub projects and infrastructure over the next 5 years (Power Mag). Lowering costs through continued innovation and scale will be key for hydrogen to succeed as an energy source long-term.
Storage and Transportation
Hydrogen poses unique challenges for storage and transportation compared to other fuels. As the lightest element, hydrogen gas is highly flammable and requires high pressure or extremely cold temperatures to achieve sufficient energy density for practical applications (source). At ambient temperatures and pressures, hydrogen has very low energy density per volume, so it needs to be compressed or liquefied for storage and transportation.
Compressing hydrogen gas to 700 bar allows it to achieve a density comparable to liquefied natural gas. However, the compression process requires significant energy. High-pressure tanks also add weight and pose safety issues in transportation applications. Alternatively, hydrogen can be liquefied at -253°C to achieve higher density, but this also requires substantial energy for cooling and super-insulated cryogenic tanks.
The difficulties and energy costs associated with compression, liquefaction, and specialized containment systems are major hurdles for feasible hydrogen storage and transportation. Advanced technologies like chemical hydrogen carriers and metal hydrides show promise but have not yet proven viable at scale. Until more efficient solutions emerge, hydrogen’s physical properties pose inherent challenges compared to liquid fuels.
Safety
Hydrogen does carry some safety concerns compared to other fuels, namely due to flammability and leakage risks. Hydrogen is highly flammable and can ignite more easily than gasoline or natural gas. However, it is important to note that while hydrogen has a low ignition energy, its flammable range is narrower than other fuels. This means it requires precise mixing with air to burn, and does not sustain a flame in open environments (Ballard).
The small size of hydrogen molecules can lead to easier leakage from storage containers. Proper materials and design must be used to minimize leakage risk. However, hydrogen rapidly dissipates and rises quickly when leaked, meaning leaks do not accumulate. Hydrogen’s light weight compared to air also prevents trapped pockets from occurring (Atlas Copco).
While hydrogen does have flammability and leakage risks, these can be properly managed through proper handling, storage, and safety systems. With proper precautions, hydrogen does not pose significantly higher safety risks than existing fuels like gasoline or natural gas.
Future Outlook
Hydrogen has the potential to play a major role in the renewable energy transition, but it also faces some limitations. According to The Future of Hydrogen Energy, demand for renewable hydrogen is expected to see steady growth through niche applications in the transportation, electricity generation, and industrial sectors. However, as discussed in Future of Hydrogen as an Alternative Fuel for Next-Generation Energy Demands, realizing hydrogen’s full potential requires overcoming challenges related to production costs, storage, transportation infrastructure, and safety.
On the positive side, hydrogen can enable greater use of intermittent renewable energy sources like solar and wind by acting as energy storage. When there is excess renewable electricity, the excess can be used to produce hydrogen through electrolysis. The hydrogen can then be stored and used later to generate electricity or power vehicles. This helps deal with the intermittency issues of renewables.
However, some experts argue that other storage technologies like batteries and pumped hydro may be more economical overall. Additionally, hydrogen’s low volumetric energy density makes storage and transportation more difficult compared to fuels like gasoline. More research and infrastructure development is needed for hydrogen to be viable as a widespread energy carrier.
While hydrogen faces obstacles, many governments and companies are investing heavily in hydrogen technology. With continued innovation and infrastructure buildout, hydrogen has the chance to grow into a major clean energy solution. But its growth is likely to be gradual and focused on specific applications rather than an outright replacement for fossil fuels.
Conclusion
In summary, hydrogen has potential as an energy source but still faces some key challenges. The main methods of hydrogen production like steam methane reforming and electrolysis using renewable electricity show promise but need to become more efficient and cost-competitive. Using hydrogen in fuel cell vehicles can reduce emissions but the infrastructure needs major build-out. Hydrogen is versatile and can be used for electricity generation and energy storage, but other renewables like solar and wind currently lead in those applications. While hydrogen is clean at point of use, total lifecycle emissions depend on production methods. Storing and transporting hydrogen safely and economically also remains a hurdle. Initial government incentives and pilot projects are moving the hydrogen industry forward, but widespread viability may still be decades away. Overall, hydrogen is a flexible energy carrier with environmental benefits if produced sustainably. With more research and investment, hydrogen could play a growing role in a clean energy future, but is not yet ready to fully replace other energy sources.