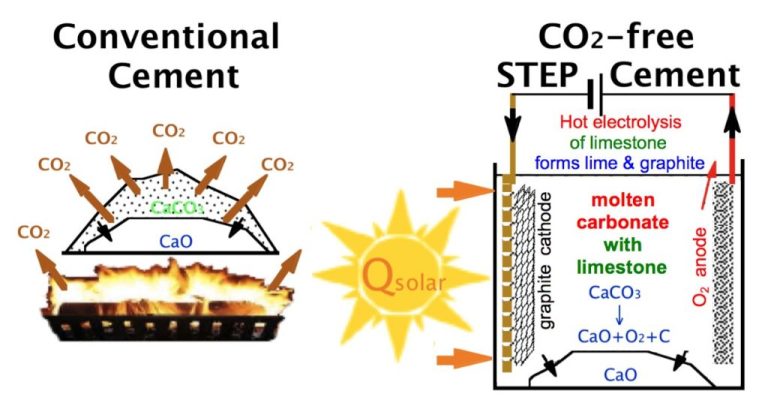
Is Energy In Matter Is Stored In Its Chemical Bonds?
What is Energy?
Energy is the capacity to do work or produce heat. It exists in many different forms that can be grouped into major categories:
Kinetic energy is the energy possessed by an object due to its motion. For example, a moving vehicle has kinetic energy.
Potential energy is energy stored in an object due to its position or chemical composition. For example, a battery has potential energy that can provide electricity.
Thermal energy is the internal energy of an object due to the motion of its atoms and molecules. Heat is the transfer of thermal energy between objects.
Chemical energy is energy stored in the bonds between atoms within molecules. Food and fuels contain chemical energy that is released in chemical reactions.
Nuclear energy is energy stored within an atom’s nucleus and released in nuclear reactions. Large amounts of energy can be released from small amounts of matter in nuclear reactions.
Energy is never created or destroyed, but it can be converted from one form to another. The ability to convert energy to do work or produce heat is what makes energy a valuable resource across the sciences and in practical applications.
What are Chemical Bonds?
Chemical bonds are attractions between atoms and molecules that form as a result of the sharing or transfer of electrons. There are different types of chemical bonds that form depending on the atoms involved:
Ionic bonds – These bonds form between a metal and a nonmetal atom. The metal atom transfers one or more electrons to the nonmetal atom, resulting in positive and negative ions that attract each other. Ionic bonds result in ionic compounds like table salt.
Covalent bonds – These bonds involve the sharing of electrons between two nonmetal atoms. The shared electrons contribute to the outer shells of both atoms. Covalent bonds can be polar or nonpolar depending on how equally the electrons are shared.
Metallic bonds – These bonds form between metal atoms. The outer electrons of metal atoms are delocalized and spread out over many positive metal ions in a “sea of electrons.” This allows for the malleability and conductivity of metals.
There are other types of chemical bonds like hydrogen bonds and van der Waals forces, but ionic, covalent, and metallic bonds are the main types that form when atoms combine to make molecules and compounds.
Energy is Stored in Chemical Bonds
Chemical bonds are what hold atoms together in molecules and compounds. These bonds form through the sharing or transfer of electrons between atoms. Forming a chemical bond requires energy, while breaking a bond releases energy. This is because energy is stored within the bonds themselves.
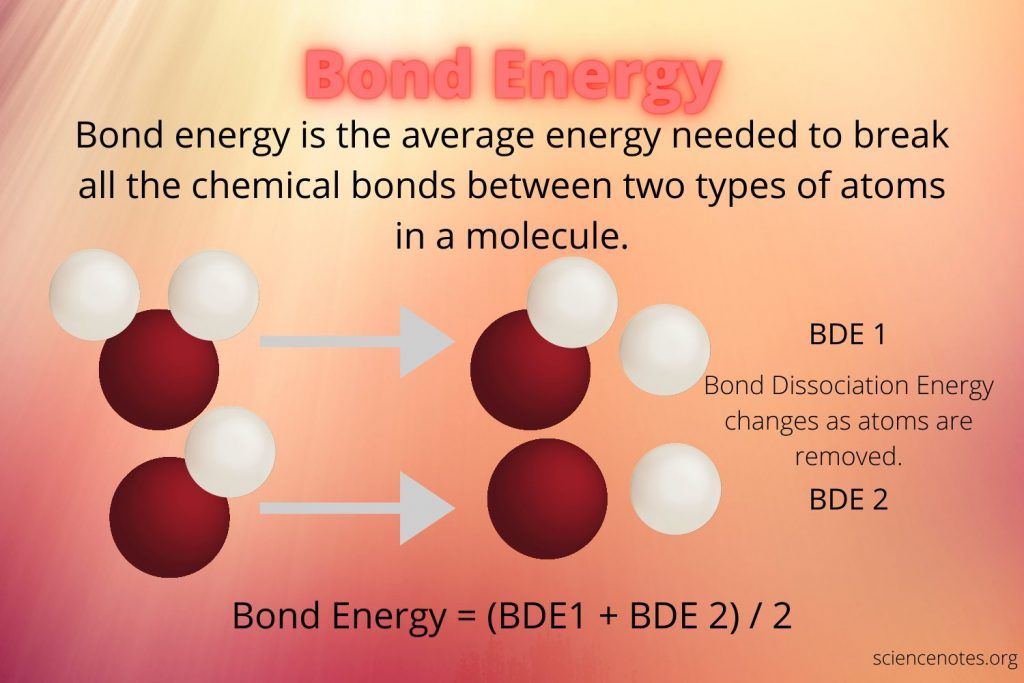
When two atoms come together to form a bond, their electrons shift into lower energy states and energy is released, similar to a ball rolling downhill. The strength of a chemical bond depends on how much energy was required to force the atoms together initially. Strong bonds like those in carbon or nitrogen require a lot of energy to overcome the repulsion between the atom’s electrons and nuclei. Therefore, a greater amount of energy is stored in these strong bonds.
The same principle applies in reverse – energy must be added to break a bond. Because it took a large amount of energy to form a strong carbon-carbon bond, that same amount of energy will be released when the bond is broken. Essentially the energy used to establish the bond gets stored within it. The more energy needed to form a bond, the more energy released when that bond breaks.
This stored energy is what fuels chemical reactions. Breaking old bonds releases energy that can then form new bonds, powering the reaction. Foods, fuels, and explosives store chemical energy that is harnessed for human purposes when their molecular bonds break.
Examples of Energy Storage
All energy exists in one form or another. For that energy to be useful, we often must be able to store it and control its release. One way that energy gets stored for later use is through chemical bonds.
For example, batteries store energy by using chemical reactions that can be reversed. In rechargeable batteries, electricity is used to drive chemical reactions that store the energy in the chemical bonds. Then when the batteries are used, these chemical reactions are reversed and release electricity.
Food energy is another example. The chemical bonds in the nutrients we eat contain energy that is released when those bonds are broken during digestion. The energy gets transferred and rearranged, but ultimately comes from the stored chemical energy in the food.
Fossil fuels like oil, gas and coal also provide energy through chemical bonds. When we burn them, the stored chemical energy gets released as heat and light.
So in many everyday instances, we observe energy that originates from chemical bonds. Lifeforms require this stored energy to survive and function, while human technologies exploit chemical energy storage for transportation, electricity, heating, cooking and more.
Measuring Bond Energy
One way to measure the energy stored in chemical bonds is through the use of a bomb calorimeter. This device allows scientists to directly measure the energy released when bonds are formed or broken in a reaction.
In a simple bomb calorimeter, the reactants are placed in a sealed metal container called the bomb. This bomb is submerged in a known volume of water inside an insulated bucket. The reaction is triggered inside the bomb, releasing energy that heats the surrounding water. By measuring the temperature increase of the water, scientists can calculate the energy released by the reaction using the known heat capacity of water.
For example, when hydrogen and oxygen gases react to form water, the energy released from the breaking and forming of bonds can be directly measured in the bomb calorimeter. From many such measurements, average bond dissociation energies have been determined for common chemical bonds:
- H-H bond: 436 kJ/mol
- O=O double bond: 498 kJ/mol
- C-C single bond: 347 kJ/mol
- C=C double bond: 611 kJ/mol
The direct calorimetric measurement of bond energies in chemical reactions confirms that energy is indeed stored within the bonds of molecules and released when those bonds are broken.
Factors Affecting Bond Energy
There are several factors that determine and influence the strength of chemical bonds, which in turn affects the amount of energy stored within those bonds.
One key factor is bond length, which refers to the distance between the nuclei of the two bonded atoms. There is an inverse relationship between bond length and bond energy – the shorter the bond length, the greater the bond energy. This is because it takes more energy to separate two atoms that are close together versus two that are farther apart.
Bond order, which indicates the number of chemical bonds between a pair of atoms, also correlates with bond energy. Bonds with higher bond orders tend to have shorter bond lengths and thus, higher bond energies. Double and triple bonds, which have bond orders of 2 and 3 respectively, exhibit greater bond energies than single bonds which have a bond order of 1.
The degree of hybridization of the orbital s involved in bonding affects bond strength and energy as well. sp hybrid orbitals overlapping head-on result in stronger sigma bonds compared to bonds formed from pure unhybridized p orbitals overlapping in a sideways fashion. Hybridization explains why carbon-carbon single bonds are stronger than carbon-carbon double bonds, for example.
Resonance stabilization weakens bonds and lowers their overall bond energies. The delocalization of pi electrons over multiple bond structures increases stability which corresponds to lower bond energy. Conjugated systems and molecules with multiple resonance structures tend to have weaker bonds compared to non-resonant structures.
In general, multiple bonds with high bond orders formed from hybrid orbitals at short bond lengths store more energy than single bonds between less electronegative atoms at longer distances.
Practical Applications
Chemical energy storage has many practical real-world applications. One of the most common is batteries, which convert the chemical energy in their active materials directly into electrical energy. Primary batteries like alkaline batteries contain all the reactants to generate electricity, while rechargeable batteries like lithium-ion batteries can have their chemical reactants restored by applying an external electrical current.
Batteries allow the portability of a wide range of electronic devices by packing chemical energy into a small, lightweight package. From smartphones to electric vehicles, battery technology enables mobility and accessibility of power. Research continues to improve battery capacity, safety, lifespan, and charging rates.
Chemical energy is also harnessed on a large scale for transportation through the metabolism of food and the combustion of fuels. The breakdown of nutrients like fats and carbohydrates provides energy for living organisms. Similarly, combustion of fuels like gasoline, diesel, and jet fuel in engines converts chemical energy into kinetic energy to propel vehicles. Understanding the energetic properties of these fuels helps improve engine and transportation systems.
Optimizing energy storage in chemical bonds has applications ranging from powering biomedical devices to reducing the environmental impact of energy usage. As research advances, chemical energy storage holds exciting potential to meet both small-scale portable power needs and large-scale energy demands.
Limitations and Challenges
While chemical bonds can store significant amounts of energy, there are some limitations and challenges to consider:
Storing large amounts of energy in chemical bonds can be difficult. Some bonds may only store modest amounts of energy, so they would need to be present in very large numbers to store the quantities needed for many practical applications. Synthesizing enough of the right chemical compounds to meet high energy demands is a major obstacle.
There is also often energy lost during the process of forming or breaking chemical bonds. Energy must be put in to break bonds, and some energy is dissipated as heat during bond formation. This makes the storage and retrieval of energy in chemical bonds less than 100% efficient. The more energy loss during these processes, the lower the net energy that can be stored and utilized.
Researchers continue to look for ways to improve the energy storage capacity of chemical bonds while also minimizing energy losses. But for now, these limitations impose challenges on how much practical energy can be stored and extracted through chemical bonds alone.
Latest Research
Scientists are actively exploring ways to improve energy storage in chemical bonds to enable advances in battery technology, renewable energy, transportation, and more. Two key areas of research are:
Improving Battery Technology
Lithium-ion batteries are the dominant battery technology today, powering everything from phones to electric cars. Researchers are developing new electrode materials, electrolytes, and battery designs to increase the energy density of lithium-ion batteries. For example, lithium-sulfur batteries can potentially hold 2-3 times more energy per pound compared to lithium-ion. Solid-state lithium batteries can improve safety by replacing liquid electrolytes with solid components. Advances in nanomaterials are also enabling higher storage capacity and faster charging times.
Biofuels Research
Biofuels provide a renewable way to store energy in chemical bonds via photosynthesis. Scientists are engineering enzymes and microbial pathways to improve the production and energy density of biofuels. For example, researchers are developing ways to optimize photosynthesis in algae and cyanobacteria to produce fuels like biodiesel and hydrogen more efficiently. Synthetic biology approaches are also being used to engineer microbes that can convert plant biomass into advanced biofuels.
Overall, research on improving energy storage in chemical bonds is critical for transitioning to renewable energy and powering a more sustainable future.
Conclusion
In summary, we’ve explored how energy is stored in the chemical bonds between atoms in molecules and compounds. This bond energy is what powers many essential processes of life, technology, and industry.
We’ve seen examples of how energy is stored in bonds like ATP and gasoline, enabling cells to function and vehicles to move. The strength of chemical bonds can be precisely measured, and many factors like bond order, length, and electronegativity determine the amount of energy stored.
Understanding bond energy has allowed scientists to better control chemical reactions and build more efficient energy storage systems. Ongoing research aims to utilize bond energy in innovative ways, from improving batteries to developing new fuels and materials.
The ability to store energy in chemical bonds is truly remarkable. It powers life’s activities and human technology, and holds potential for meeting our future energy needs in sustainable ways.