Is Battery A Type Of Energy Source?
A battery is a portable energy storage device that converts chemical energy into electrical energy. Batteries are composed of one or more electrochemical cells that have positive and negative electrodes known as the cathode and anode. When a battery is connected to an external load, a redox reaction occurs where electrons flow from the anode to the cathode through the load, generating an electric current. This makes batteries a convenient way to store and transport energy for powering a wide variety of devices.
The purpose of this article is to explain what batteries are, how they work, the different types of batteries, and their advantages and limitations compared to other energy sources. We will also look at battery safety considerations and provide an overview of the latest battery research and future technologies.
How Batteries Work
Batteries are devices that store chemical energy and convert it into electrical energy. They consist of one or more electrochemical cells, each containing a positive electrode (cathode), a negative electrode (anode) and an electrolyte that allows ions to move between the electrodes.
During discharge, the anode undergoes a chemical oxidation reaction that produces electrons. These electrons flow through the external circuit to the cathode, producing an electric current. Meanwhile, the cathode undergoes a chemical reduction reaction that consumes electrons. The electrolyte provides ions to facilitate the reactions. The electrons want to move from the anode to the cathode, creating an electrical potential that powers the device.
When recharging, an external power source applies a voltage that reverses the electrochemical reaction. The cathode releases electrons while the anode consumes them, causing the ions to move back through the electrolyte to their original locations.
In summary, batteries convert the potential energy stored in chemical bonds into electrical energy during discharge. Recharging reverses the process to rebuild the chemical bonds and store more energy.
Types of Batteries
There are several major types of batteries used today for a variety of applications:
Lead-Acid Batteries
Lead-acid batteries are one of the oldest and most widely used battery technologies. They use leads plates immersed in a diluted sulfuric acid electrolyte solution. Lead-acid batteries are commonly used in vehicles and other large applications where weight is not a major concern. They have lower energy density than other battery types but are inexpensive and can provide high surge currents.
Lithium-Ion Batteries
Lithium-ion (Li-ion) batteries use lithium ions that move from the negative to the positive electrode during discharge. They have become very popular in recent years for consumer electronics because of their high energy density, lack of memory effect, and slow self-discharge when not in use. Li-ion batteries are also increasingly used in electric vehicles and energy storage systems.
Nickel-Cadmium Batteries
Nickel-cadmium (NiCd) batteries use nickel oxide hydroxide and metallic cadmium as electrodes. They have higher energy density than lead-acid batteries and are known for their durability and ability to discharge quickly. However, cadmium is toxic and NiCd batteries have been replaced in many applications by nickel-metal hydride (NiMH) and lithium-ion batteries.
Batteries vs Other Energy Sources
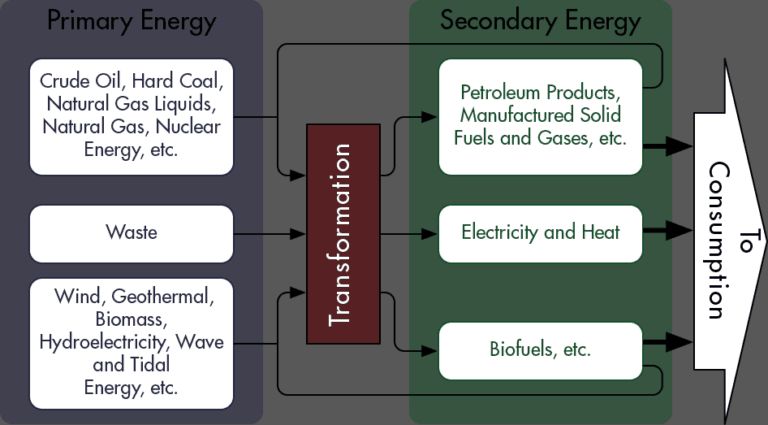
Batteries differ from other energy sources like fossil fuels, solar, wind, and nuclear in some key ways:
Portability: Batteries can store energy and release it on demand, making them highly portable. Other energy sources like fossil fuels, solar, wind, and nuclear require either an engine or power plant to convert the energy into electricity. This limits their mobility and portability compared to batteries.
Energy Density: The amount of energy stored per unit volume or weight is called energy density. Batteries generally have lower energy densities than fossil fuels, though research is improving this. Nuclear energy has by far the highest energy density.
Dispatchability: Batteries can dispatch energy on demand, while solar and wind depend on environmental conditions. Nuclear and fossil fuels can dispatch power more easily than batteries with the right infrastructure.
Emissions: Batteries produce no direct emissions. Fossil fuels emit greenhouse gases and air pollutants when burned. Nuclear has low emissions. Solar and wind have no emissions during operation.
Cost: Nuclear and fossil fuels benefit from economies of scale. Battery costs are falling but remain higher than these traditional sources. Solar and wind costs have also declined dramatically in recent years.
In summary, batteries offer unique advantages like portability and emissions-free operation. But they also face limitations around energy density and cost compared to other sources. Continued research aims to improve batteries’ competitiveness as an energy storage solution.
Advantages of Batteries
Batteries offer several key advantages compared to other energy sources that have made them an essential technology in modern society. Some of the main benefits of using batteries include:
Portability
One of the biggest advantages of batteries is that they allow energy to be stored in a portable, self-contained unit. Unlike fossil fuels which require bulky infrastructure like power plants and grid transmission lines, batteries can pack a lot of energy into a small, lightweight package. This makes batteries ideal for powering portable consumer electronics like laptops, phones, and power tools that need to be able to run on the go.
Rechargeability
Many types of batteries are designed to be recharged and reused hundreds or thousands of times. Rechargeable batteries can continually store and provide energy as needed, then be replenished by plugging them into an external power source like the grid. This is much more sustainable and cost-effective than disposable batteries or one-time fuel sources.
Low Maintenance
Once installed, batteries require little regular maintenance compared to other energy systems like generators or fuel cells that have many moving parts requiring upkeep. Modern batteries are mostly “install and forget” devices with self-contained, sealed components that don’t need refueling or much user intervention beyond occasional charging.
Limitations of Batteries
While batteries offer many advantages as energy sources, they also come with some key limitations that need to be considered.
One major limitation of batteries is their capacity. Most conventional batteries like lead-acid or nickel-metal hydride have a relatively low energy density compared to fossil fuels. This means a battery of equivalent energy capacity to a tank of gasoline would take up far more weight and space. The driving range of electric vehicles, for example, is limited by current battery technology’s energy density. Improvements in energy density are being made, but remain a limiting factor.
Batteries can also be quite costly, especially lithium-ion batteries which require rare earth metals. Large battery packs add substantial expense to products like electric vehicles and energy storage systems. Bringing down these costs through improved manufacturing and new battery chemistries is an ongoing challenge.
There are also environmental concerns around batteries. Many contain toxic metals and chemicals that require proper recycling and disposal. Mining the raw materials, particularly lithium, also raises sustainability issues. Work is being done to develop more eco-friendly batteries using abundant, non-toxic materials.
In summary, capacity, cost, and environmental impact are key drawbacks of batteries that technology aims to address. But these limitations currently restrict the applications of batteries and prevent widespread adoption as energy sources.
Battery Safety
Batteries can pose safety hazards if not handled, stored, disposed of, or recycled properly. Here are some tips for battery safety:
Proper Handling:
- Avoid exposing batteries to extreme heat or cold.
- Do not crush, puncture, or disassemble batteries.
- Do not let battery terminals touch each other or other metals.
- Avoid touching leaking or damaged batteries.
- Keep batteries away from small children.
- Handle and use lithium-ion batteries with caution – they can overheat and ignite.
Safe Storage:
- Store batteries in a cool, dry place.
- Keep batteries in original packaging until use.
- Separate batteries from other metals when storing.
- Place tape on the terminals if storing batteries outside of a device.
Proper Disposal and Recycling:
- Many communities have battery recycling programs – use them instead of throwing batteries in the trash.
- Some retailers accept batteries for recycling – check with local stores.
- Do not burn or incinerate batteries as they contain toxic materials.
- Bring damaged or leaking batteries to a hazardous waste disposal facility.
Following basic battery safety practices helps prevent fires, explosions, leaks, and exposure to hazardous materials.
Latest Battery Research
Researchers around the world are working to develop new and improved battery technologies. Here are some of the most promising innovations in battery research:
Solid-state batteries – These replace the typical liquid or gel-based electrolyte with a solid, conductive material. This makes the batteries more stable and less prone to overheating. Solid-state batteries could potentially pack 2-3 times more energy into the same size battery. Major companies like Toyota and Volkswagen are actively developing solid-state batteries for electric vehicles.
Lithium-air batteries – Lithium-air batteries draw in oxygen from the air to enable an electrochemical reaction. This gives them a much higher energy density than lithium-ion batteries. There are still challenges to overcome around their stability and ability to be recharged. If perfected, lithium-air batteries could store more than 5 times as much energy per pound compared to lithium-ion.
Aluminum-air batteries – Aluminum is highly energy dense and much more abundant and cheaper than lithium. Aluminum-air batteries use aluminum as the anode and oxygen from the air as the cathode. This combination offers up to 10 times the energy density of lithium-ion batteries. Work is being done to try to make rechargeable aluminum-air batteries practical and affordable.
Sodium-ion batteries – Sodium is abundant and cheap compared to lithium. Sodium-ion batteries could offer comparable performance to lithium-ion at a much lower cost. Chemists are working to develop suitable electrodes and electrolyte materials to make sodium-ion batteries viable and efficient.
Flow batteries – Flow batteries pump liquid electrolytes through an electrochemical cell to store energy. This makes it easy to scale up their storage capacity. Flow batteries last longer than solid batteries and can discharge fully without damage. Researchers are working to improve their energy density and bring down their cost.
Future of Battery Technology
The future looks bright for innovations in battery technology. Here are some exciting areas of research and development:
New battery chemistries – Scientists are exploring new materials and chemical processes that could lead to major gains in energy density, charging speed, safety, and cost. For example, lithium-sulfur and zinc-air batteries show promise.
Solid state batteries – These replace liquid electrolytes with solid components, eliminating potential leakage or flammability issues. Solid state batteries could enable lighter and more powerful batteries.
Graphene batteries – Graphene’s superconductive properties may allow batteries to charge in seconds. Graphene could also lead to improved capacitance and voltage.
3D-printed batteries – Additive manufacturing can produce batteries in unique shapes and embed them into devices and structures. It also enables rapid prototyping.
AI-designed batteries – Machine learning and computational modeling can discover new battery materials and compositions faster than lab experiments.
Wireless charging – Longer-range wireless charging would enable our devices to charge without cables or pads. This could lead to improvements in convenience, safety, and waterproofing.
Advances in batteries will enable applications like electric planes, smarter wearable devices, highly efficient electric cars, and grid-scale energy storage. The future is bright for better batteries powering our world.
Conclusion
Batteries are a form of energy storage, not energy generation. While batteries rely on chemical reactions to produce electricity, they do not create energy themselves. Batteries simply store energy from another source, like solar or wind power, and release it on demand.
The key takeaways are:
- Batteries convert chemical energy into electrical energy using electrochemical reactions.
- Common types of batteries include lead-acid, lithium-ion, nickel-cadmium, and alkaline.
- Compared to fossil fuels, batteries can store energy from clean sources and power devices efficiently without emissions.
- Limitations of batteries include capacity, charging time, cost, and safety concerns.
- Ongoing research aims to improve battery life, charging speed, materials, and sustainability.
In summary, while extremely useful as energy storage devices, batteries are not primary energy sources like coal, oil, natural gas, or renewable sources such as solar and wind. Batteries require another energy source to initially charge them. However, they provide an efficient, clean way to store energy for on-demand use in many modern applications.