How Is Work Related To The Change In Energy?
Work and energy are two fundamental concepts in physics that are closely related. Work involves transferring energy from one place or system to another through the application of a force. Work is done when an object is moved over a distance by an external force acting on the object. Energy comes in different forms such as kinetic energy, potential energy, thermal energy, and more. The work-energy theorem states that the net work done on an object is equal to its change in kinetic energy.
In this article, we will explore the definition of work, its units, the work-energy theorem, everyday examples, and the relationship between work and energy in fields like thermodynamics and physiology.
Definition of Work
In physics, work is defined as the transfer of energy by a force acting on an object to move it over a distance. It is calculated as the product of the force (F) applied on the object and the displacement (d) of the object in the direction of that force:
Work = Force x Displacement
W = Fd
The units of work are joules (J) in the SI system. One joule is defined as the work done by a force of one newton moving an object one meter in the direction of the force. Work is a scalar quantity, meaning it has magnitude but no direction.
The work done on an object results in a transfer of energy to the object. More specifically, the work causes a change in the object’s kinetic energy, potential energy or internal energy. So work is a mechanism of transferring energy between objects through the action of forces.
Units of Work
In physics, work is measured in units called joules. A joule is defined as the work done by a force of one newton over a distance of one meter. This means that the SI unit for work is the newton-meter (N∙m) or joule (J).
One joule is equal to the amount of work done by a 1-newton force moving an object 1 meter. Some examples are:
- Lifting an apple 1 meter straight up would require about 1 joule of work.
- Lifting a textbook 1 meter straight up would require about 10 joules of work.
- Pushing a chair across a floor for 3 meters against a friction force of 10 newtons would require about 30 joules of work.
In summary, the joule is the standard unit used to quantify work in physics and other scientific fields. The joule allows precise measurement and comparison of the amount of work done in various mechanical systems.
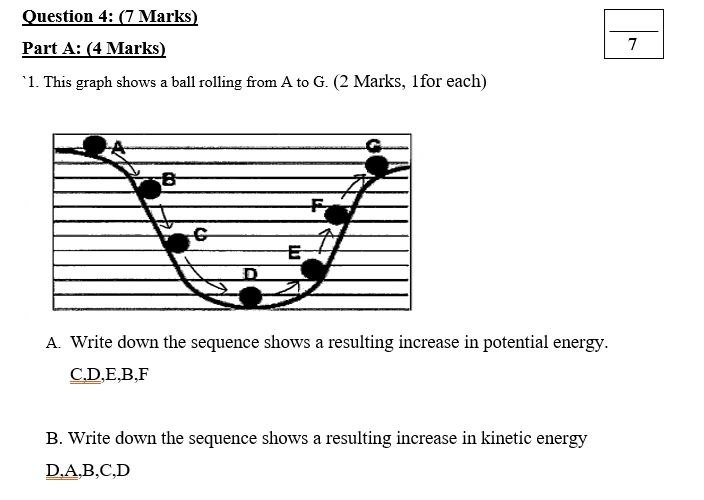
Work-Energy Theorem
The work-energy theorem is one of the most important concepts in physics. It relates the work done on an object to its resulting change in kinetic energy.
The theorem states that the net work done on an object equals its change in kinetic energy. In equation form:
Wnet = ΔK
Where:
- Wnet is the net work done on the object
- ΔK is the change in the object’s kinetic energy
This theorem reveals that work done on an object transfers energy to the object, increasing its kinetic energy. This transferred energy manifests as an increase in the object’s speed and motion.
Some key points about the work-energy theorem:
- The net work equals the change in kinetic energy, not the total kinetic energy.
- The theorem considers only work done by external forces, not internal ones.
- It applies to the net work done by all external forces, not just individual forces.
The work-energy theorem has crucial implications and applications in physics and engineering. It provides a powerful tool for analyzing mechanical systems involving work and energy transfer.
Examples
Physics problems often illustrate the concept of work with simplified scenarios. Here are some example problems showing how to calculate work:
A person pushes a crate across a floor with a force of 50 Newtons. The crate moves a distance of 5 meters. The work done is calculated as:
Work = Force x Distance
Work = 50 N x 5 m
Work = 250 Joules
A ball is lifted vertically upwards with a force of 10 Newtons. The ball rises 2 meters. The work done lifting the ball is:
Work = Force x Distance
Work = 10 N x 2 m
Work = 20 Joules
These simplified scenarios demonstrate how to calculate the work done by multiplying the applied force by the distance moved in the direction of the force. Real-world situations may involve additional complexities, but the underlying work-energy principle remains the same.
Power
Power is defined as the rate at which work is done. It is calculated as work divided by time. The units for power are joules/second, which is also called a watt. The more work you can do in a shorter amount of time, the more power you exert.
Here are some examples of power:
- A factory machine that can package 100 boxes per hour is exerting more power than one that can only package 50 boxes per hour.
- A car with a more powerful engine capable of going from 0-60 mph in 5 seconds exerts more power than a car that takes 10 seconds to reach that speed.
- Athletes like sprinters exert a lot of power during short bursts of intense activity.
As you can see, power is an important concept related to work and how quickly it can be done. The rate of doing work is power.
Work in Thermodynamics
In thermodynamics, work refers to the transfer of energy between a system and its surroundings due to the change in internal energy of a system. The thermodynamic theory relating to work is based on the law of conservation of energy which states that energy cannot be created or destroyed. When a system undergoes a thermodynamic process, its internal energy changes. This change in internal energy can be quantified as work done on or by the system.
The work done during a thermodynamic process depends on the path between the initial and final states. For reversible processes, the work done is equal to the change in internal energy of the system. However, for irreversible processes, the work done is always smaller than the change in internal energy due to energy dissipation. The maximum useful work that can be extracted from a system undergoing a process between two states is equal to the change in the Gibbs free energy of the system.
In thermodynamic processes involving heat transfer, the amount of useful work that can be done is directly related to the quantity of heat transferred. This relationship is governed by the second law of thermodynamics, which introduces the concept of entropy. Entropy is a measure of the disorder of a system. Natural processes always proceed in the direction of increasing entropy. Therefore, there is a limit to the amount of useful work that can be obtained when heat is transferred between bodies at different temperatures.
To summarize, work plays a key role in thermodynamics by providing a link between the macroscopic parameters of a system and the microscopic energy transfers occurring at the molecular level. Calculating work done helps quantify processes like heat engines, refrigerators, and chemical reactions in terms of the first law of thermodynamics.
Work in Physiology
In the human body, the muscles perform mechanical work to move the limbs and body. This requires energy expenditure. The amount of mechanical work done by muscles determines the energy requirement for that movement.
For example, when walking up stairs, the leg muscles contract to lift the body against gravity. More mechanical work is done compared to walking on a flat surface. Thus, walking up stairs requires more energy expenditure by the leg muscles. This extra energy comes from cellular respiration in the muscle cells, where ATP is generated by burning fuels like glucose and fats.
We can calculate the mechanical work done by muscles using force measurements during limb movements. This gives an estimate of energy expenditure for that activity. Higher mechanical work and force generation by muscles leads to more energy requirement and higher calorie burn.
This relationship between mechanical work and energy expenditure allows exercise physiologists to quantify the metabolic costs of various physical activities. It also helps explain why some exercises like weightlifting or sprinting require more energy than others like walking or swimming.
Overall, the human body converts chemical energy from food into mechanical work and movement. Optimizing mechanical efficiency allows us to move using minimal energy expenditure.
Everyday Examples
The concepts of work and energy are integral to our everyday lives. Here are some examples of how these physics principles apply to common human activities:
Exercising: When you lift weights, go for a run, or do any other type of exercise, your muscles are doing work. They apply force over a distance as you move the weights or your body. This requires your muscles to use energy, which comes from the food you eat. The more intense the exercise, the more work is done and energy expended.
Climbing stairs: Walking up a flight of stairs requires your legs to do work against gravity. They apply force to lift your body upwards against the downward force of gravity over the vertical distance of the stairs. More energy is expended climbing stairs compared to walking on flat ground.
Manual labor: Jobs like construction, farming, and forestry require a lot of physical work. Shoveling, hammering, lifting, and carrying objects over distances mean the workers constantly do work and use energy in their muscles. The intensity of the labor determines how much energy they expend each day.
Typing: Even small finger movements require your muscles to do work. Typing on a keyboard involves applying force over short distances as your fingers press the keys. The energy comes from carbohydrates and fats metabolized in your body.
So in our daily activities, our bodies are literally “work machines” that apply force and use energy to accomplish tasks!
Conclusion
In conclusion, work and energy are intrinsically linked through the work-energy theorem, which states that the net work done on an object is equal to its change in kinetic energy. We explored how work is defined as a force applied over a distance, with SI units of joules. Work can cause transfers between different forms of energy, such as kinetic, potential, thermal, or chemical energy.
We looked at examples of how work applies in physics, thermodynamics, physiology, and everyday life. Whether it’s calculating the work needed to accelerate a car, do mechanical work like lifting objects, perform chemical work during digestion, or any other process, work leads to a change in the object’s energy.
Understanding the relationship between work and energy allows us to quantify processes in our universe and harness them to our advantage. Work and energy are important concepts with many applications in science, engineering, and society.