How Is Heat Energy Transferred?
Heat energy is the total kinetic energy and potential energy of molecules within a substance. Thermal energy refers specifically to the kinetic energy associated with molecular motion. Heat transfer is the process by which thermal energy is exchanged between substances or systems, due to a temperature difference. Understanding heat transfer is crucial for a variety of applications including engineering, materials science, chemistry, physics, biology, and more.
Studying how heat transfer occurs allows us to predict and control the flow of thermal energy for purposes such as heating and cooling systems, insulation, electronics, cooking, and so on. It is particularly important in fields like mechanical engineering and construction, where managing heat flow can impact efficiency and safety. Properly accounting for conduction, convection, radiation, and phase changes enables effective design of systems from jet engines to buildings. Overall, comprehending heat transfer principles and mechanisms grants us tremendous control over our thermal environment.
Conduction
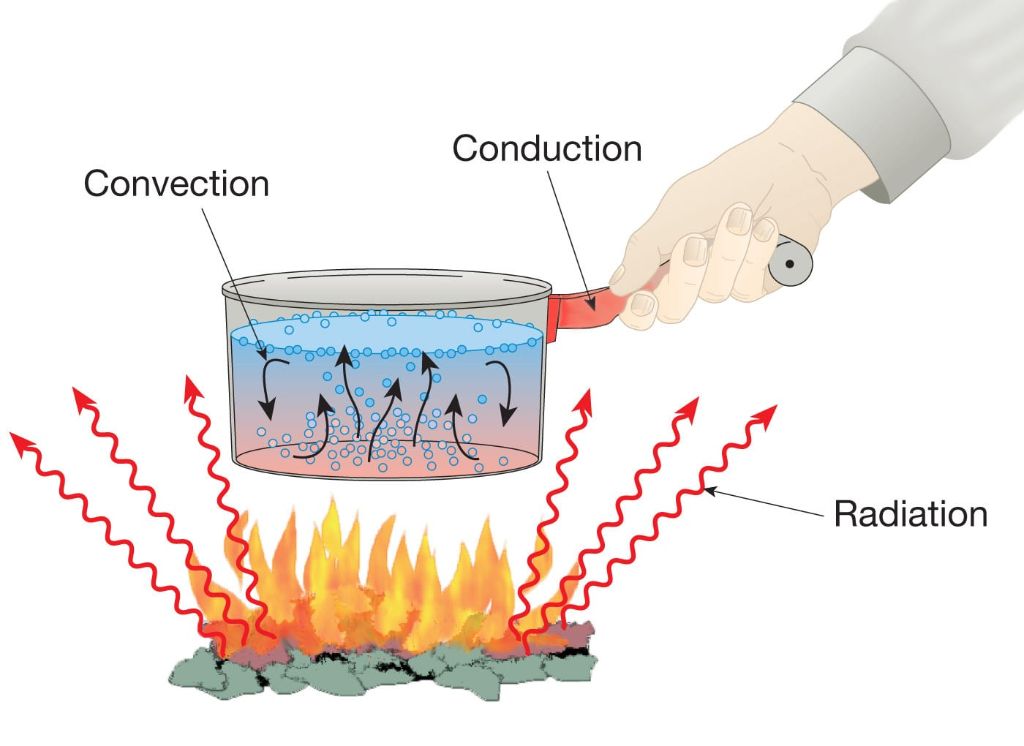
Conduction is the transfer of heat energy through direct contact between neighboring molecules and atoms [1]. As molecules and atoms collide, the more energetic ones transfer kinetic energy to the less energetic ones, with energy propagating from regions of higher temperature to lower temperature [2]. This process occurs readily in solids, which have their atoms and molecules close together, but less so in gases, where the molecules are far apart [3].
On a molecular level, conduction works through vibrations and collisions. In a solid, the atoms or molecules vibrate in place. As they vibrate, they collide with their neighbors, transferring kinetic energy. The neighbors in turn collide with their neighbors, propagating the energy transfer through the material [1]. The more energetic atoms vibrate more vigorously, colliding into their neighbors with greater force and transferring more kinetic energy. This is why heat energy flows from high temperature to low temperature regions during conduction.
Examples of conduction include a spoon heating up when placed in hot soup. The metal atoms comprising the spoon collide with energetic soup molecules, gaining kinetic energy that we perceive as heat. Another example is a pot heating up on a stove – the metal pot bottom conducts heat from the hot stove surface to the rest of the pot.
Convection
Convection is the transfer of heat energy through the movement of fluids, such as liquids or gases. As the fluid is heated, it expands and becomes less dense than the surrounding fluid. This causes the heated fluid to rise due to buoyancy while the cooler fluid sinks down to take its place. This movement and circulation of fluids is called a convection current.
For example, when a pot of water is heated on the stovetop, the water at the bottom of the pot becomes hot first. As it heats up, it expands, becomes less dense, and rises to the top. This heated water is replaced by cooler water from the middle and bottom of the pot that sinks and is heated in turn. The circular movement of the water distributes heat from the bottom of the pot throughout the liquid (cite: https://study.com/academy/lesson/what-are-convection-currents-definition-examples-quiz.html).
Convection currents also occur in the atmosphere as air is heated by the Earth’s surface. For example, air near the equator gets heated up and rises, creating large convection cells that transport heat energy from the equator to the poles. The cooler air then sinks back down at the poles and flows along the surface back toward the equator, creating wind currents (cite: https://homework.study.com/explanation/how-do-convection-currents-work.html). Convection currents play a major role in weather patterns and ocean currents.
Radiation
Thermal radiation refers to the emission of electromagnetic waves from all matter that has a temperature greater than absolute zero. Objects and surfaces emit thermal radiation constantly. The hotter something is, the more thermal radiation it emits at shorter wavelengths. This radiation does not require a medium to transfer heat. Thermal radiation emitted by an object is directly proportional to the fourth power of its absolute temperature, as described by the Stefan-Boltzmann law.
Thermal radiation transfers heat in the form of electromagnetic waves, which can travel through empty space. These waves have a wide range of wavelengths and frequencies, including visible light, ultraviolet light, infrared radiation, microwaves, and radio waves. The wavelength distribution and intensity of the radiation depends on the temperature of the emitting object. Hotter objects tend to emit radiation with shorter wavelengths.
When thermal radiation is absorbed by another object, it converts the radiation energy into thermal energy, thus heating that object. For example, we feel warmth from the thermal radiation emitted by the sun, and the earth emits thermal radiation that escapes into space. Greenhouse gases in the atmosphere absorb some of this infrared radiation, trapping heat and causing global warming. Thermal radiation does not require direct contact between source and destination objects, which distinguishes it from conduction and convection. Radiative heat transfer plays a major role in astronomy and space science.
Conduction vs Convection vs Radiation
Conduction, convection and radiation are the three main ways that heat energy is transferred from one place to another. While they share similarities, there are important differences between the three heat transfer mechanisms:
| Type | Description |
|---|---|
| Conduction | Heat transfer through direct contact between materials. Heat energy is transferred between particles of a substance as the more energetic particles collide with less energetic ones.
Examples: Heat traveling through a solid pan from the stove burner to the handle, heat moving through a metal spoon placed in hot soup. |
| Convection | Heat transfer via movement of fluids. Heat energy is carried between places by the bulk motion of a fluid like air or water.
Examples: Hot air rising from a radiator, currents forming in heated liquid. |
| Radiation | Heat transfer through electromagnetic waves/photons. Heat in the form of infrared radiation is emitted by hot objects and absorbed by cooler ones.
Examples: Heat from the sun warming the Earth, heat radiating from hot coals in a fireplace. |
A key difference is that conduction requires physical contact between materials, convection relies on bulk fluid motion, and radiation works across space. Conduction is generally the most efficient, followed by convection, while radiation is usually much slower but can happen across empty space since it does not require direct contact or a transport medium like a fluid. Understanding how heat is transferred in different situations allows effective thermal management through material choices and design.
Real-World Examples
Heat transfer is occurring all around us in nature and in our everyday lives. Here are some common examples:
The heating of buildings and homes relies heavily on heat transfer. Furnaces and boilers use conduction to transfer heat from burning fuel into air or water, which is then distributed throughout the building through convection [1]. Insulation in walls and windows reduces heat loss to the outdoors through conduction.
Cooking food also involves multiple forms of heat transfer. Stovetops and ovens use conduction to transfer heat into pots and pans, which then transfer heat into food through conduction. As food cooks, hotter molecules transfer heat to cooler ones through conduction. Steam rising from a pot involves heat transfer through convection [2].
The heating of the Earth by the Sun occurs through radiation. The Sun radiates thermal energy across space, which passes through the atmosphere and is absorbed by the Earth’s surface [3]. This heat energy is then distributed around the globe through convection in the atmosphere and oceans.
Animals, including humans, utilize conduction, convection and radiation to exchange heat with their surroundings and maintain proper body temperature.
Applications
Heat transfer principles have a wide range of applications in engineering and technology. Some key examples include:
Thermal management of electronics – Controlling heat dissipation is critical in microchips and other electronics to prevent overheating. Heat sinks, fans, and other cooling methods rely on conduction, convection, and radiation principles. Rohsenow (1985)
HVAC systems – Heating, ventilation, and air conditioning rely on heat transfer to control temperatures in buildings and vehicles. Heat exchangers are designed using heat transfer calculations. Rohsenow (1985)
Power generation – Power plants like nuclear, coal, and concentrating solar plants require careful heat transfer analysis to convert thermal energy into electricity efficiently. Principles and Applications of Heat Transfer in Engineering (2023)
Chemical processing – Reactors, distillation columns, boilers, and condensers all depend on heat transfer principles for design and optimization. This includes managing energy balances and phase changes.
Automotive engineering – Engine cooling, exhaust systems, HVAC, and emission controls in vehicles involve heat transfer considerations for optimum design.
Measuring Heat Transfer
The rate of heat transfer can be measured using several methods and equations. The main factors that determine heat transfer rate are the temperature difference between two objects or environments, the conductive and convective properties of the materials involved, and the surface area over which the heat is being transferred.
For conduction, the rate of heat transfer Q is calculated using Fourier’s Law: Q = kA(ΔT/Δx) where k is the thermal conductivity, A is the cross-sectional area, ΔT is the temperature difference, and Δx is the thickness of the conductive material. This equation can be used to measure heat flowing through solid objects like walls or pipes. Thermocouples placed at two points can measure the temperature difference.
For convection, Newton’s Law of Cooling gives the rate of heat transfer between a surface and a fluid: Q = hA(Ts – T∞) where h is the convective heat transfer coefficient, A is the surface area, Ts is the surface temperature, and T∞ is the bulk fluid temperature. Convection coefficients can be looked up for standard scenarios, and thermocouples measure the two temperatures.
For thermal radiation, the Stefan-Boltzmann law provides the heat transfer rate: Q = εσAT14 – T24 where ε is emissivity, σ is the Stefan-Boltzmann constant, A is the surface area, T1 is the first surface temperature, and T2 is the second surface temperature. Infrared thermometers can measure surface temperatures.
Overall, by carefully measuring temperatures, surface areas, material properties, and temperature differences, these equations allow quantitative measurement of heat transfer rates through conduction, convection, and radiation.
Influencing Factors
There are many factors that can affect the rate of heat transfer. Some of the main ones include:
Significant factors affecting heat transfer performance of vapor chambers: A critical review – This article discusses factors like wick structure, heat source, filling ratio, space height, working fluid, and more.
Temperature difference – Heat flows from high temperature to low temperature, so a greater temperature difference between two objects leads to faster heat transfer.
Thermal conductivity – Materials like metals have high thermal conductivity and transfer heat quickly, while insulators transfer heat slowly.
Surface area – More surface area in contact allows for faster heat transfer.
Fluid movement – Moving fluids transfer heat faster through convection.
Thickness – Thicker materials impede heat transfer compared to thinner ones.
Type of medium – Heat transfers at different rates through solids, liquids, and gases.
Radiation exposure – More exposure to electromagnetic radiation increases radiative heat transfer.
Conclusion
In summary, heat energy is transferred in three main ways: conduction, convection, and radiation. Conduction is the transfer of heat between substances that are in direct contact with each other, like a spoon getting hot on a stove. Convection is the transfer of heat by the movement of heated fluid or gas, like hot air rising or water boiling. Radiation is the transfer of heat in the form of electromagnetic waves that can travel through space, like the warmth from the sun.
Understanding the different methods of heat transfer allows us to control heat flow for many applications – from designing efficient building insulation to high-performance cooling systems for computers. It also helps explain natural phenomena like wind patterns and the ocean’s impact on climate. With a solid grasp of heat energy transfer, we can make the most of this useful form of energy in smart and innovative ways.