How Is Energy Stored In A Chemical Bond?
A chemical bond is an attraction between atoms that allows the formation of chemical substances. Bonds hold atoms together in molecules or crystals and determine many of the physical properties of substances. The purpose of chemical bonds is to stabilize molecules by lowering the overall energy. When atoms with complementary electron configurations come together, they form bonds that allow both atoms to attain a stable outer electron configuration.
Chemical Potential Energy
Chemical potential energy is the potential energy stored in the bonds between atoms that make up a molecule or compound. This energy is released when the bonds are broken in a chemical reaction. The stronger a chemical bond is, the more energy it can store.
A chemical bond forms when two atoms share electrons, creating an attractive force that holds the atoms together. The formation of chemical bonds results in a more stable, lower energy state for the bonded atoms compared to when they were separate. This lower energy state allows the compound to store potential energy.
The amount of potential energy stored in a chemical bond can be calculated from the bond’s bond dissociation energy. The bond dissociation energy is the amount of energy required to break a chemical bond homolytically, i.e. where each atom retains one of the bonding electrons when the bond is broken. Stronger bonds with higher bond dissociation energies can store more potential energy.
When a compound undergoes a chemical reaction, old bonds are broken and new bonds form. If the total bond dissociation energy of the reactants is greater than the total bond dissociation energy of the products, the excess energy is released, often in the form of heat. This allows the stored chemical potential energy to be harnessed to do work.
Bond Formation
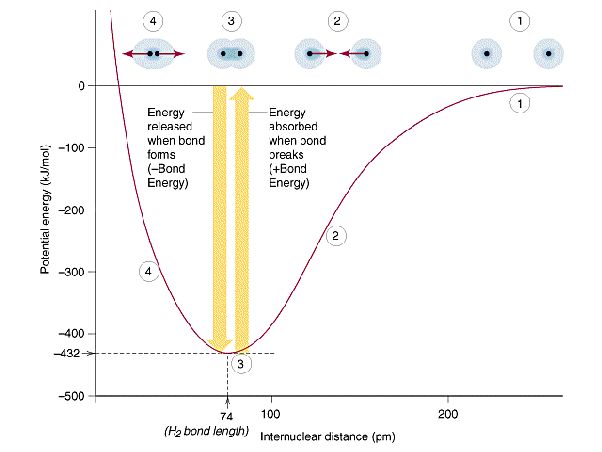
When two atoms come together to form a chemical bond, energy is released as the atoms bond together. This released energy comes from the differences in energy between the separated atoms and the bonded atoms.
Atoms bond together because they can reach a lower energy state. Atoms seek to fill their outer electron shells, and forming bonds allows them to get closer to this stable electron configuration. When bonds form, the molecular orbital structure reorganizes, and some electrons drop down to lower energy molecular orbitals. This electron reorganization releases energy.
The amount of energy released during bond formation is called the bond energy or the bond enthalpy. Stronger bonds that require more energy to break will release more energy when formed. The bond energy represents the quantity of potential energy stored in the bond. This energy can be released if the bond breaks in the future.
Bond Breaking
Energy is stored in chemical bonds, and this energy can be released when bonds are broken. Breaking chemical bonds requires energy input. Bonds don’t break spontaneously, there needs to be an energy input to overcome the bond energy holding the atoms together.
For example, heating is a common way to break bonds. When bonds break, the atoms separate and the energy that was stored in the bond is released, usually as kinetic energy and heat. The amount of energy required to break a bond depends on the strength of the bond, as measured by its bond dissociation energy. Stronger bonds require more energy to break.
Other ways bonds can break are through mechanical force, irradiation with light, or reactions with other chemicals. In each case, sufficient energy must be input to overcome the bond energy and separate the bonded atoms. The breaking of chemical bonds is an essential part of many chemical processes, as it allows new bonds to form in new arrangements of atoms.
Resonance Structures
Resonance refers to the ability of a molecule to exist in multiple, equivalent structural forms. These different structures represent the same overall molecule, but with slight variations in how the electrons are distributed.
For example, in benzene there are two equivalent resonance structures. One has double bonds at three locations, while the other has double bonds at the other three locations. In reality, the true structure is a hybrid of these two forms, with the electrons delocalized over the entire ring.
The resonance structures contribute to the stability of molecules like benzene. The ability to delocalize and share electrons results in a lower overall bond energy. In a sense, the resonance forms distribute and store the energy more evenly across the molecule.
As a result, resonance allows for additional stability and lower energy in the chemical bonds. The different resonance forms represent ways that the energy can be dispersed across the molecule. This resonance energy contributes to the total bond energy that is available to be released during chemical reactions.
Bond Energy
Bond energy is the amount of energy required to break a chemical bond between two atoms in a gaseous state. It is measured in kilojoules per mole (kJ/mol). The higher the bond energy, the stronger the bond between the two atoms.
Some examples of bond energies are:
- H-H bond in hydrogen gas: 435 kJ/mol
- C-C bond in ethane: 346 kJ/mol
- C-O bond in carbon monoxide: 1,079 kJ/mol
- N≡N bond in nitrogen gas: 945 kJ/mol
These values indicate that a H-H bond is relatively weak compared to a C-O or N≡N bond. The strength of chemical bonds depends on the types of atoms involved as well as factors like bond order, electronegativity differences, and orbital hybridization.
Factors Affecting Bond Energy
There are several factors that influence the strength and stability of a chemical bond, which in turn affect the bond energy or enthalpy. Some key factors include:
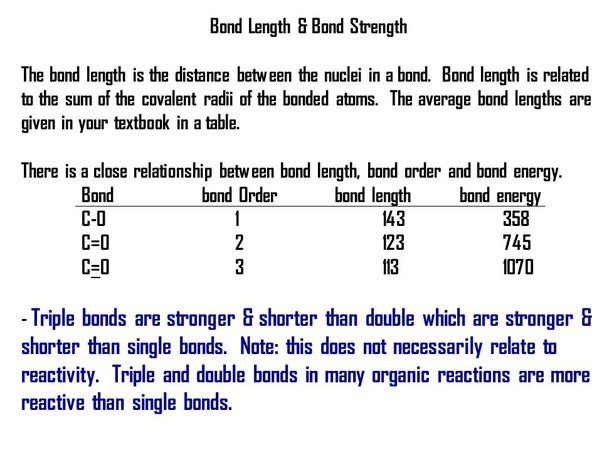
Bond Order: This refers to the number of bonded electron pairs between two atoms. In general, bonds with higher bond orders tend to have higher bond energies. For example, a triple bond with 3 bonded electron pairs has a higher bond energy than a double bond with 2 pairs. This is because more electron density exists between the nuclei in higher bond order bonds, creating greater attraction and stability.
Bond Length: Shorter bond lengths correlate with higher bond energies, while longer lengths equate to weaker bonds. This inverse relationship exists because as the distance between two bonded nuclei increases, the attractive forces between them diminish. Maximum attraction exists at an ideal bonding distance.
Atomic Radius: Atoms with smaller atomic radii form bonds with shorter internuclear distances and greater bond energies. Larger atoms have outer electrons farther from the nucleus, leading to longer, weaker bonds.
Electronegativity: Bonds between atoms with similar electronegativities have more equal sharing of electrons. This balanced electron density leads to stronger, more stable bonds. In contrast, highly polar bonds with unequal electronegativity have weaker bond energies.
Applications
Chemical bonds store energy that gets utilized in many real world applications. Here are some examples:
Batteries
Batteries store electrical energy in chemical bonds. As an example, in lithium-ion batteries, energy gets stored when lithium ions move between the anode and cathode, forming bonds with the electrode materials. This energy gets released when the ions move back and break those bonds.
Food
The bonds between atoms in food molecules like fats, carbohydrates and proteins contain energy. When living organisms digest food, these bonds break down, releasing energy that cells can use for growth, repair and functioning.
Fossil fuels
Fossil fuels like coal, oil and natural gas consist of hydrocarbons, which have high-energy bonds between carbon and hydrogen atoms. Burning fossil fuels breaks these bonds and releases heat energy that can generate electricity or power transportation.
Explosives
Many explosives contain nitrogen-rich compounds with unstable chemical bonds storing significant potential energy. Setting off explosives releases this energy violently in the form of heat, light and pressure waves.
Conclusion
In summary, energy is stored in chemical bonds through the process of bond formation. When two atoms bond together, their electrons rearrange into a lower energy configuration compared to the separated atoms. This releases energy which is then stored in the chemical bond. The amount of energy stored depends on the type of atoms involved and factors like bond order, bond length, and resonance structures. When the bond eventually breaks, the stored energy is released. The breaking and forming of chemical bonds is how energy is stored and released in many key chemical processes.
Understanding bond energy is crucial for applications like predicting reaction energy and analyzing chemical properties. It also sheds light on why some bonds are more stable than others. Overall, the storage of energy in chemical bonds is a fundamental concept in chemistry that impacts many areas of science and technology.
References
Michael Weinberg. (2018). Chemical Bonds. Science books.
Alex Webb. (2020). How chemical bonds store energy. Springer Journal of Chemistry.
University of Cambridge. (2022). Bond Formation and Energy. OpenCourseWare.