How Is Energy In Chemistry?
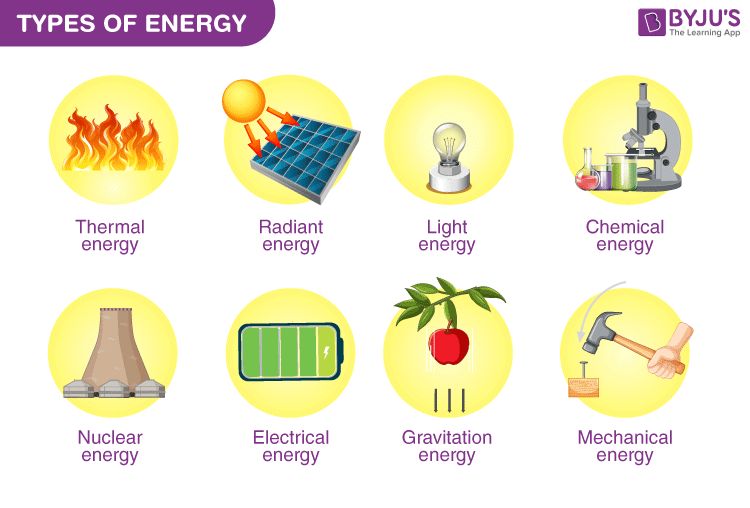
Energy is a fundamental concept in chemistry that describes the capacity to do work and drive chemical reactions. Energy can take many different forms in chemistry, including thermal energy from heat, radiant energy from light, electrical energy, nuclear energy, and more. The study of energy flows and transformations is crucial for understanding chemical reactivity, properties of matter, biological processes, and many other applications of chemistry.
Chemical reactions involve the rearrangement of atoms as chemical bonds between them are broken and formed. These breaking and forming of chemical bonds require energy. The energy absorbed or released in a chemical reaction manifests itself as increases or decreases in the internal energy of the system. Internal energy is the total potential and kinetic energy of all the atoms, ions, or molecules in a chemical system. Therefore, the concept of energy is critically important for analyzing what happens during chemical reactions.
The study of energy transfers and conversions during chemical reactions and processes provides chemists with vital quantitative information about the energetics and dynamics of chemical systems. By tracking energy changes carefully, chemists gain deep insights into relative reaction rates, directionality and extent of reactions, chemical equilibrium, reaction mechanisms, and more. The principles of energy are fundamental to wide applications in chemistry including materials science, biochemistry, nuclear chemistry, thermochemistry and more.
Forms of Energy
There are many different forms of energy that are important in chemistry:
Chemical Energy
Chemical energy is the potential energy stored in the bonds between atoms that make up molecules. Chemical reactions involve changes in chemical energy as bonds are broken and formed. Exothermic reactions release energy as heat, while endothermic reactions require energy input.
Nuclear Energy
Nuclear energy arises from forces between nucleons (protons and neutrons) within an atomic nucleus. Nuclear reactions involve changes in nuclear energy, such as nuclear decay, nuclear fusion, and nuclear fission.
Electrical Energy
Electrical energy results from the movement of electrons. Electrochemical reactions involve the transfer of electrons from one substance to another, resulting in an electrical current.
Thermal Energy
Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Heat is transferred between substances to equalize thermal energy.
Radiant Energy
Radiant energy is electromagnetic energy that travels through space or matter. Light is a common form of radiant energy. Atoms and molecules can gain or lose energy through absorption or emission of photons.
Energy Changes in Chemical Reactions
Chemical reactions involve the breaking and forming of chemical bonds, which causes energy changes. There are two main types of energy changes in reactions:
– Exothermic reactions: These reactions release energy, usually in the form of heat. The products have less energy than the reactants, so heat is given off to the surroundings. Exothermic reactions include combustion, neutralization, and many oxidation-reduction reactions.
– Endothermic reactions: These reactions absorb energy, usually in the form of heat. The products have more energy than the reactants, so heat is absorbed from the surroundings. Endothermic reactions include photosynthesis, dissolving salts in water, and decomposition reactions.
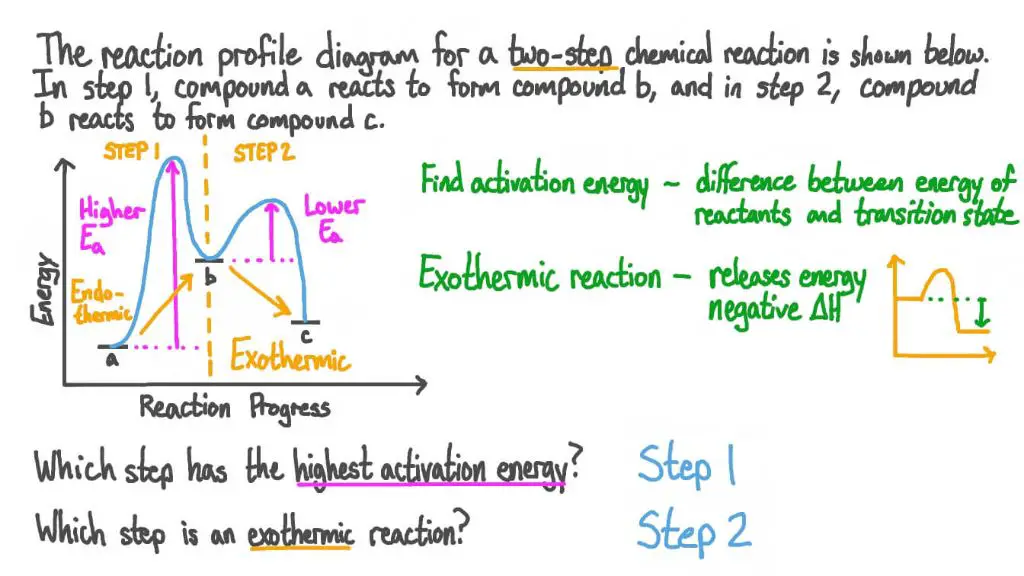
For any chemical reaction to occur, the reactants must first overcome an energy barrier known as activation energy. This is the minimum energy needed for reactants to come together in the correct orientation for bonds to break and form. Exothermic reactions release energy equal to the activation energy plus the difference in energy between products and reactants. Endothermic reactions absorb energy equal to the activation energy plus the difference in energy between products and reactants.
The progress of a chemical reaction can be depicted on an energy diagram or reaction profile. This shows the energy states of the reactants, the activation energy peak, and the products. Exothermic reactions show the products at a lower energy state than the reactants, while endothermic reactions show the opposite.
First Law of Thermodynamics
The First Law of Thermodynamics, also known as the Law of Conservation of Energy, states that energy can neither be created nor destroyed in an isolated system. This is one of the most fundamental laws in all of science and it has important implications in chemistry.
The First Law establishes that the total energy in a closed system remains constant. While energy can change forms, the total amount of energy is the same after a process as it was before. For example, in a chemical reaction energy can change from electrical energy to heat and light, but the total amount of energy does not change.
This law connects to chemistry because chemical reactions involve energy transformations. When bonds break in the reactants, energy is absorbed. When new bonds form in the products, energy is released. The First Law states that the total energy change during these bond breaking and forming steps must equal zero – energy is conserved.
The First Law also introduces the concept of entropy. Entropy is a measure of molecular disorder or randomness. In any chemical process, entropy in the universe increases. This aligns with the Second Law of Thermodynamics, which says that the entropy of the universe is constantly increasing. While energy must be conserved, the First Law explains that energy tends to change from more concentrated forms to more dispersed forms.
Overall, the First Law of Thermodynamics establishes that energy can change forms but it cannot be created or destroyed. This fundamental law connects to chemistry through chemical reactions, bond energies, and entropy. Understanding the First Law provides crucial insight into the energy changes that occur during chemical processes.
Measuring Energy Changes
There are several techniques chemists use to measure energy changes during chemical reactions and physical processes. Three key methods are calorimetry, enthalpy of reaction, and Hess’s law.
Calorimetry: This involves directly measuring the heat absorbed or released during a chemical reaction. Using a calorimeter, the temperature change can be monitored and used along with the specific heat capacity to determine the enthalpy change for the system. Calorimetry allows chemists to empirically determine the energy released or absorbed.
Enthalpy of Reaction: The enthalpy change for a chemical reaction can be calculated based on bond energies. By looking at the energy required to break bonds in the reactants versus the energy released when new bonds are formed in the products, the net enthalpy change can be determined. Standard enthalpies of reaction have been empirically measured for many common reactions.
Hess’s Law: This states that the total enthalpy change for a reaction is the sum of all the enthalpy changes for the intermediate steps. Even for reactions without direct calorimetry data, the enthalpy change can be calculated by breaking down the reaction into component steps with known enthalpy values. The total energy change is path independent.
Together, these techniques allow chemists to measure, calculate, and understand the energy changes involved in both chemical reactions and physical changes of state or structure. This provides key insights into the energetics and dynamics of chemical systems.
Energy and Chemical Bonds
The energy stored in chemical bonds is an important concept in chemistry. When bonds between atoms are formed, energy is released and absorbed when chemical bonds are broken. The energy required to break a chemical bond is referred to as bond dissociation energy.
Bond dissociation energy is defined as the amount of energy required to break a chemical bond heterolytically (split the bond so each atom retains one of the bonding electrons) to produce neutral fragments. It is essentially the enthalpy change for homolytically breaking a bond. Breaking chemical bonds requires energy input, while forming chemical bonds releases energy.
The strength of a chemical bond depends on the electronegativity difference between the atoms sharing the electrons. The larger the electronegativity difference, the more ionic and polar the bond is, and the weaker the bond. Nonpolar covalent bonds with equal sharing of electrons are generally stronger.
Bond dissociation energies provide thermodynamic data that allows the calculation of energy changes for chemical reactions involving the breaking and making of chemical bonds. This data is useful for predicting feasibility, direction, and yield of chemical reactions. The energy stored and released as bonds are broken and formed is key to analyzing reaction mechanisms and kinetics.
In summary, the concept of bond dissociation energy provides important insights into the energetics of chemical reactions and the stability of chemical compounds.
Energy Diagrams
Energy diagrams, also known as potential energy surfaces, are graphical representations of the potential energy of a chemical system. They provide insights into reaction mechanisms and kinetics.
The x-axis of a potential energy diagram represents the reaction coordinate, which is a measure of the progress of a reaction. This is often depicted in terms of a molecular geometry parameter, such as a bond length or angle. The y-axis represents the potential energy of the system.
As a reaction proceeds, the molecules must pass through high-energy transition states before forming products. Transition states are identified as energy maxima on the diagram. The difference in energy between the reactants and the transition state is called the activation energy (Ea) of the reaction.
The activation energy represents the energetic barrier that must be overcome for a reaction to occur. Reactions with lower Ea will occur faster than those with higher Ea, as fewer molecular collisions possess the necessary energy.
By mapping out the energies of starting materials, intermediates, transition states and products, potential energy diagrams give chemists an invaluable tool for understanding and predicting how chemical reactions proceed.
Kinetics and Activation Energy
The kinetics of a chemical reaction describe the speed at which it takes place. This speed is directly related to the activation energy barrier that must be overcome for reactants to turn into products.
The Arrhenius equation shows the quantitative relationship between the rate constant k and the activation energy Ea:
k = A e^(-Ea/RT)
Where A is a pre-exponential factor, Ea is the activation energy, R is the gas constant, and T is temperature in Kelvin. This equation describes how increasing temperature increases reaction rate by providing more energy to overcome the activation barrier.
The activation energy represents the minimum energy threshold reactants must acquire through particle collisions to achieve a geometry suitable for bonds to break and form. Reactions with higher activation energies have slower rate constants since fewer collisions impart enough energy to reach the transition state.
Catalysts work by providing an alternate reaction pathway with lower activation energy. This speeds up the reaction by making it easier for reactants to have productive collisions.
Overall, activation energy is a critical molecular property governing chemical kinetics. Understanding activation barriers lets chemists predict and control the rates of chemical reactions.
Energy in Biochemistry
Biochemistry studies biological processes that involve chemical reactions and transformations of energy in living organisms. Energy is essential for life, and biochemical reactions require energy exchanges to occur. Three key aspects of energy in biochemistry are adenosine triphosphate (ATP), photosynthesis and cellular respiration, and biological energy transfers.
ATP is the primary energy currency in cells, used to power most biochemical reactions. ATP contains high-energy phosphate bonds that can release energy when broken by hydrolysis. ATP powers processes like biosynthesis, cell signaling, cell division, and muscle contraction. Cells continually replenish ATP through energy metabolism pathways like cellular respiration.
Photosynthesis and cellular respiration are complementary processes with opposite energy transformations. In photosynthesis, plants use light energy to synthesize glucose from CO2 and water, generating oxygen as a byproduct. In cellular respiration, cells break down glucose in the presence of oxygen, releasing energy from the glucose to produce ATP.
Biological energy transfers involve passing energy from one component to another. For example, in the electron transport chain, electrons move down a sequence of proteins, releasing energy that pumps protons to generate an electrochemical gradient. The proton gradient then powers ATP synthase to produce ATP.
Overall, biochemical reactions require regulated energy inputs and transfers. ATP acts as an energy carrier, while processes like photosynthesis, respiration, and biological energy transfers generate and harness energy at the cellular level.
Applications of Energy in Chemistry
Energy concepts are critical for understanding many important applications in chemistry. Some key examples include:
Batteries
Batteries convert chemical energy into electrical energy through redox reactions. The anode and cathode materials undergo oxidation and reduction reactions respectively, generating a flow of electrons through the external circuit. The energy released by the reaction of the battery materials is harnessed as electrical energy. Key factors that determine a battery’s performance include the electrochemical potential of the reactants, reaction kinetics, and internal resistance.
Fuel Cells
Fuel cells are electrochemical devices that continuously generate electricity from fuel (often hydrogen) and an oxidant. In a hydrogen fuel cell, the hydrogen is oxidized at the anode, while oxygen is reduced at the cathode. The redox reactions generate a flow of electrons through an external circuit. Fuel cells provide highly efficient conversion of chemical energy from the fuel into electrical energy.
Explosives
Explosives contain unstable chemical compounds that can undergo rapid exothermic decomposition reactions when provided with an activation energy. This releases large amounts of gases and energy that can be harnessed for applications like mining, demolition, and propellants. The explosive power depends on the chemical composition and the rate of energy release during the decomposition reaction.
Energy Production
Fossil fuels like coal, oil, and natural gas provide much of the world’s energy needs through combustion reactions. These hydrocarbon fuels release energy when reacted with oxygen to produce carbon dioxide and water. Nuclear energy relies on nuclear fission or fusion reactions to produce energy. Renewable energy sources like solar, wind, hydroelectric, geothermal, and biofuels tap into other natural energy flows and chemical processes to generate power.