How Does Hydrogen Energy Work
Introduction
Hydrogen energy refers to the use of hydrogen as a clean and sustainable fuel source. Hydrogen is considered an energy carrier, like electricity, rather than a primary energy source. When fed into a fuel cell, hydrogen reacts with oxygen to produce electricity, with water and heat as the only byproducts. Unlike fossil fuels, hydrogen does not emit harmful greenhouse gases when used as a fuel.
Hydrogen has a wide range of applications across the transportation, power generation, and industrial sectors. As a transportation fuel, hydrogen can power fuel cell electric vehicles with zero tailpipe emissions. Hydrogen fuel cells can provide clean power for buildings and data centers. The industrial sector can also use hydrogen for high-temperature heating and chemical processes. When produced from low-carbon energy sources like renewable electricity, hydrogen has the potential to decarbonize major parts of the energy system.
This article provides an overview of how hydrogen energy technologies work, from production and storage to end-use applications. It also discusses the future potential of hydrogen energy to help address climate change and air pollution while meeting the world’s growing energy demands in a sustainable way.
Hydrogen as an Energy Carrier
Hydrogen is considered an energy carrier, not a primary energy source. This means hydrogen is a medium that stores and transports energy in a usable form from one place to another [1]. Hydrogen has the ability to carry, store, and deliver usable energy. Other common energy carriers include electricity, gasoline, and natural gas.
For example, electricity carries energy from power plants to homes and businesses. Gasoline stores chemical energy and delivers it to vehicle engines when combusted. Natural gas pipelines transport usable energy in gaseous form from wells to end users. Similarly, hydrogen can store energy derived from renewable sources and deliver it to fuel cells to generate electricity.
The key difference is that hydrogen is not found freely available in nature like fossil fuels. It must be produced from other energy sources in processes such as electrolysis or steam methane reforming. Once produced, hydrogen’s role is to store and distribute energy in a usable way [2].
Hydrogen Production Methods
The most common and versatile method for producing hydrogen is hydrogen production through electrolysis of water, which involves passing electric current through water to separate hydrogen and oxygen atoms. This can be done using either renewable energy or fossil fuels to generate the electricity.
Another major hydrogen production method is natural gas reforming, also known as steam methane reforming. This involves reacting steam at high temperatures with methane from natural gas to produce hydrogen and carbon monoxide. The carbon monoxide can then be further reacted with steam to produce more hydrogen via the water-gas shift reaction.
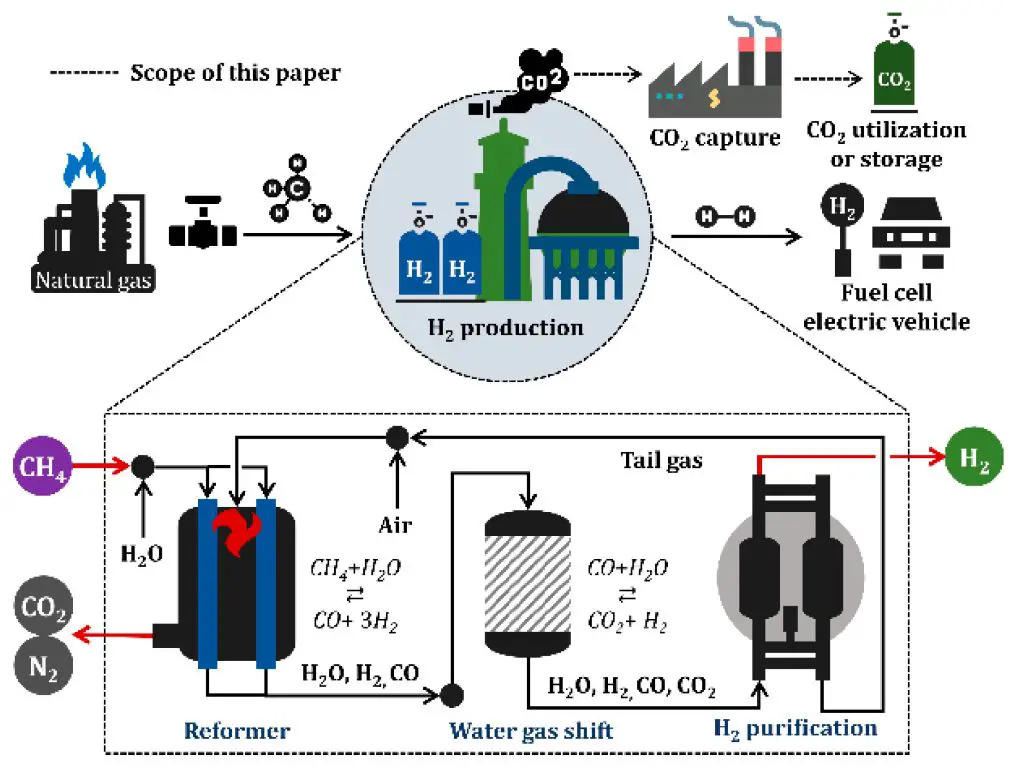
Some other hydrogen production methods being developed include thermochemical production from biomass or fossil fuels, photobiological production from algae and cyanobacteria, and photoelectrochemical production using solar energy. These methods are still in early research and development stages.
Overall, the main ways to produce hydrogen fall into electrolysis of water, steam reforming of natural gas, and novel thermochemical, photobiological, and photoelectrochemical methods using renewable energy sources.
Hydrogen Storage
Hydrogen has a low volumetric energy density, which presents challenges for storage and transportation. There are several ways hydrogen can be stored:
Compressed hydrogen gas is the most common method for storage. Hydrogen is compressed and stored in high-pressure tanks at pressures up to 700 bar. This allows for higher energy density storage, but requires heavy, expensive tanks. According to the review by Usman et al., compressed hydrogen only has a gravimetric storage density of up to 5 wt%.
Liquid hydrogen is another storage method where hydrogen is cooled to -253°C to liquify it. This increases density but requires complex insulation and energy to keep it cold. Liquefaction also causes some energy loss. According to Usman et al., the maximum gravimetric density achieved for liquid hydrogen storage is 7 wt%.
Materials-based storage encompasses adsorption by porous materials, metal hydrides, and chemical hydrides. These materials can reversibly adsorb and store hydrogen with high volumetric densities under mild temperatures and pressures. Metal hydrides can achieve over 7 wt% gravimetric density but tend to be heavy. Chemical hydrides also have high volumetric density and can store over 10 wt% hydrogen by weight.
Fuel Cells
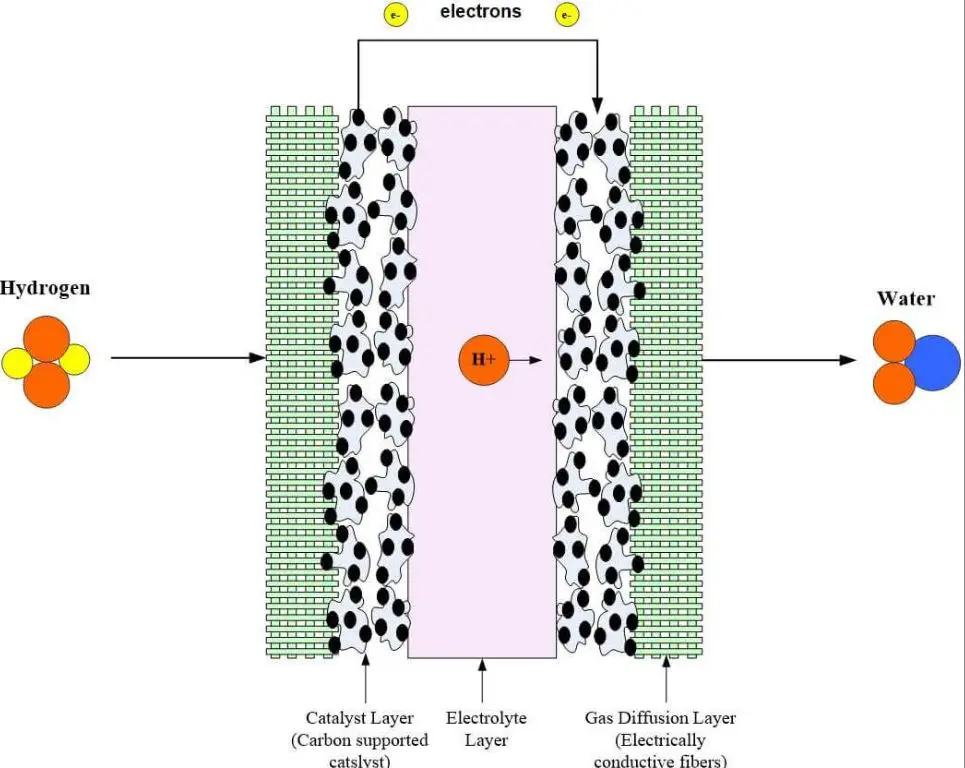
Fuel cells are electrochemical devices that convert the chemical energy of hydrogen into electricity through a chemical reaction. Hydrogen is fed into the anode side of the fuel cell, while oxygen is fed into the cathode side. At the anode, the hydrogen molecules are split into protons and electrons. The protons pass through a membrane to the cathode side, while the electrons are forced through an external circuit, generating an electric current. At the cathode, the protons, electrons, and oxygen combine to form water as the waste product. The flow of electrons through the external circuit provides the electricity that can be used to power devices or fed into the grid.
Fuel cells are very efficient at extracting energy from hydrogen, with efficiencies reaching 60% or higher. They can provide power for a wide range of applications, from small portable devices to vehicles to large stationary power generators. Unlike combustion engines, fuel cells generate electricity directly through electrochemical reaction rather than heat and mechanical energy. This makes them quieter, cleaner, and more reliable. Fuel cells will likely play a major role in enabling hydrogen to become a widespread energy carrier of the future.
Hydrogen Internal Combustion Engines
Hydrogen internal combustion engines (Hydrogen ICE) are modified versions of traditional gasoline engines that use hydrogen as a fuel instead of gasoline. The basic components are similar, with pistons, a crankshaft, valves, and a spark plug ignition system. However, modifications need to be made to handle the different properties of hydrogen compared to gasoline.
Hydrogen has a very wide flammability range, so the engines require lower compression ratios to avoid pre-ignition. The lower compression means the engines have lower efficiency than gasoline engines. Materials also need to be upgraded to handle hydrogen’s propensity to embrittle metals. The hydrogen is injected into the cylinder just before ignition to optimize combustion. Engines can be designed optimally for hydrogen from the start, but many hydrogen internal combustion engines are converted from existing gasoline engines (Cummins, 2022).
The main emission from hydrogen internal combustion engines is water vapor. There are no carbon emissions. However, NOx emissions can be higher than gasoline engines. Exhaust gas recirculation and catalytic converters help reduce NOx to make the total emissions very low. Overall, hydrogen internal combustion engines have the major advantage of eliminating carbon emissions while leveraging existing engine technology and infrastructure.
Hydrogen internal combustion engines are being developed by major manufacturers like Cummins for use in heavy duty vehicles like trucks and buses. Light duty passenger cars could also adopt the technology. Hydrogen internal combustion engines allow continued use of familiar drivetrains while providing a pathway to zero-emissions transportation (Cummins, 2022).
Hydrogen Infrastructure
One of the major challenges with developing a hydrogen economy is building out the infrastructure required to transport and store hydrogen. Compared to existing infrastructure for fossil fuels like gasoline and natural gas, hydrogen infrastructure is still in its infancy.
Hydrogen needs to be produced at large centralized facilities and then distributed to end users. Building an extensive network of pipelines to transport hydrogen would require major capital investments. Currently there are over 1,600 miles of hydrogen pipelines in the U.S., but this is dwarfed by the more than 3 million miles of natural gas pipelines (Ayers, 2012).
Beyond pipelines, transporting hydrogen via trucks, trains, barges, and ships would require new high-pressure tanks and containers. Refueling stations equipped to handle hydrogen fueling are limited as well. According to the Department of Energy, there are only 48 publicly accessible hydrogen stations in the U.S. compared to about 150,000 gas stations.
Hydrogen storage is another challenge. Hydrogen has very low volumetric energy density as a gas, so it needs to be compressed or liquefied for storage. This requires energy-intensive processes. New technologies for storing hydrogen such as metal hydrides and nanocarbon materials are being developed to try to overcome these challenges (Offshore Engineering Challenges, 2023).
Major investments into hydrogen infrastructure will be needed for it to become a widespread energy source. But overcoming these infrastructure hurdles will be key to unlocking hydrogen’s potential.
Hydrogen Safety
Hydrogen has very unique properties that make it quite safe to use as an energy carrier. Hydrogen is non-toxic and dissipates rapidly when released, reducing risk of combustion. Indeed, hydrogen has a very low ignition energy, requiring 4x more energy to ignite than gasoline. However, hydrogen’s volatility also requires proper handling and infrastructure to ensure safe transport and storage. Hydrogen’s light weight and buoyancy means any leaked hydrogen will quickly rise and disperse rather than pool on the ground. Proper ventilation standards in enclosed hydrogen facilities are critical. Overall, hydrogen is considered safer than gasoline and natural gas given its non-toxicity and quick dissipation when released. With proper safety protocols for handling, infrastructure, and applications, hydrogen energy can be implemented quite safely.
According to https://merlin-h2.com/energyh/about-hydrogen-energy/, hydrogen fuel is often incorrectly seen as unsafe due to past incidents, but has since been proven safe when proper safety measures are taken.
Hydrogen Applications
Hydrogen is emerging as an important energy carrier in the transition to renewable energy sources. It can be used across multiple sectors via fuel cell electric vehicles, fuel cell buses, fuel cell forklifts, heavy duty freight, and even passenger planes. Examples of real-world hydrogen applications in the U.S. include over 50 hydrogen refueling stations in California, hydrogen buses in multiple states, and over 30,000 forklifts powered by hydrogen fuel cells (https://www.energy.gov/eere/fuelcells/articles/hydrogen-and-fuel-cells-101).
The Hydrogen Europe 2024 conference outlines many real world hydrogen applications across hard-to-abate sectors like steel and cement production, as well as mobility applications like aviation, shipping, trucks, and passenger cars (https://events.reutersevents.com/renewable-energy/hydrogen-europe/agenda). Conferences and organizations like this highlight the growth in commercial viability of hydrogen technologies across many global industries.
As clean hydrogen production scales up and costs decline, we can expect to see more and more applications utilizing hydrogen for energy services like transportation, electricity generation, and industrial processes. The unique properties of hydrogen make it highly versatile and many experts see it as a critical part of the global energy transition.
Future of Hydrogen
The potential for hydrogen as a clean, renewable energy source is promising. Hydrogen has the highest energy density of any fuel and only emits water as a byproduct when used. Many experts predict hydrogen will play a major role in the global clean energy transition. According to a 2022 study published in MDPI Energies, “The future of hydrogen energy systems’ existence is more likely to be built on more sustainable forms of energy and raw materials (such as sun and water)” (source). With continued technological advancements and infrastructure investments, hydrogen could account for up to 25% of global energy consumption by 2050.
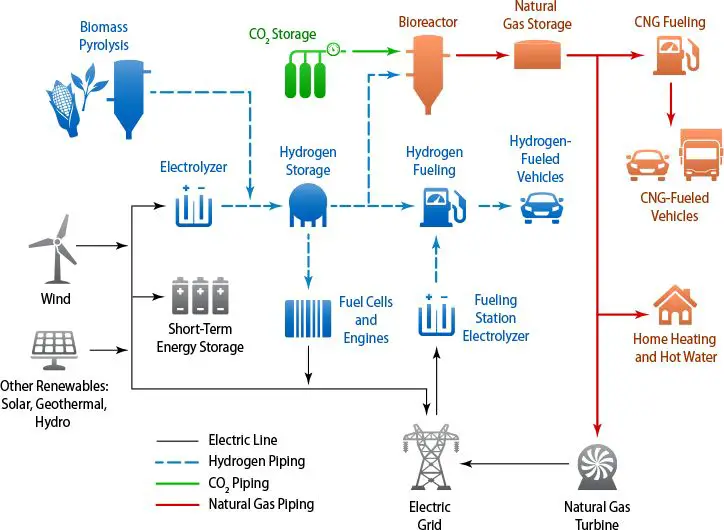
Some key advantages that make hydrogen’s future look bright: it can be produced from water using renewable electricity, stored and transported at high densities, and used in fuel cells or internal combustion engines with zero carbon emissions. Hydrogen fuel cell vehicles are already on the market, and many countries have launched hydrogen roadmaps to scale up production and infrastructure. The modular nature of hydrogen production and the potential to convert natural gas networks to carry hydrogen also aid adoption. Challenges remain around bringing down costs and concerns about leakage minimizing efficiency, but many industry and government stakeholders are aggressively pursuing solutions.
Overall, hydrogen is poised to grow as a versatile, clean energy carrier and fuel source. According to the European Institute for Innovation and Technology’s Climate-KIC, “Hydrogen is important for achieving net-zero emissions as it could eliminate 80 gigatons of CO2 by 2050.” (source) With continued progress, hydrogen promises to play a major role in decarbonizing multiple sectors and enabling the global energy transition.