How Does Heat Affect Matter In Many Ways?
Heat is a form of energy that causes atoms and molecules to vibrate faster and spread apart. When heat is applied to matter, it can induce a wide range of changes across various scales. From driving weather systems to changing material properties, heat impacts matter in many important ways that shape our world. Understanding heat’s diverse effects is crucial for fields like physics, chemistry, biology, meteorology, engineering, and more. This article will provide an overview of the key ways heat fundamentally transforms matter through thermal expansion, changes in state, enabling chemical reactions, altering material properties, affecting biological systems, transferring energy, and influencing climate.
Thermal Expansion
When heated, the molecules and atoms in all states of matter start moving more and vibrating faster. This increased motion pushes the molecules and atoms slightly further apart. As the distance between molecules and atoms increases, the overall volume of the material expands.
Solids typically expand the least with heat, while gases expand the most. However, the expansion is proportional to temperature change in both cases. Even small temperature changes in solids can lead to measurable increases in length or volume.
The expansion of materials with heat has many important real-world applications. For example, thermometers rely on the expansion of liquids like mercury or alcohol to measure temperature changes. Gaps are left between rail road tracks and bridges to allow for thermal expansion on hot days. Plumbers add bends and flexible joints to prevent pipes from buckling. Concrete roads have space between slabs to expand in the summer heat without cracking.
Change of State
Heating and cooling can change matter between solid, liquid, and gas states through melting, boiling, freezing, and condensing. When a solid is heated, its molecules gain kinetic energy and vibrate more, weakening the intermolecular bonds and causing the solid to melt into a liquid. If the liquid is heated further, the molecules gain enough energy to completely break intermolecular bonds and escape as a gas in the process of boiling or vaporization.
The reverse processes occur when cooling matter down. As a gas is cooled, it condenses into a liquid state as the molecules lose kinetic energy and are attracted together by intermolecular bonds. Further cooling causes the liquid to freeze into a solid state as thermal motion decreases and the molecules arrange into fixed positions in a rigid structure. These state changes of melting, boiling, freezing, and condensing are examples of how heating and cooling can transform matter between solid, liquid, and gas phases.
Chemical Reactions
Heat provides the activation energy needed for many chemical reactions to occur. The molecules and atoms in the reactants must collide with enough energy to break bonds and form new bonds to create the products. Heat supplies this activation energy, allowing endothermic reactions that absorb heat to proceed.
One example is cooking, where heat causes complex chemical changes that transform ingredients into tasty foods. Applying heat breaks down plant and animal tissues, caramelizes sugars, and drives the Maillard reaction that produces new flavors. Another example is combustion engines like those in cars and power plants. The heat of combustion powers the engine while also enabling a series of reactions that convert fuel into exhaust.
The rate of chemical reactions generally increases with temperature, as heat provides more molecular collisions with enough energy to react. However, too much heat can denature enzymes and other proteins that serve as catalysts for reactions. Overall, heat is a crucial driver of chemical reactions, powering many important processes in everyday life.
Material Properties
The strength, hardness, and ductility of materials can change significantly with temperature. As temperature increases, the atomic vibrations in a material become more energetic, which can weaken interatomic bonds. This generally causes the material’s strength and hardness to decrease as temperature rises. However, some materials like carbon steel become more ductile at higher temperatures as the crystal structure changes to allow dislocations to move more easily.
The dependence of material properties on temperature is critical in material science and manufacturing applications. For example, annealing involves heating a metal to make it more ductile for further processing. Workability of metals and alloys strongly depends on temperature. Heat treatment can tailor the properties of alloys by altering their microstructure. Thermal processing like quenching can create internal stresses that increase strength. The maximum service temperature of materials limits high temperature applications.
Material properties at low temperatures are also important. Some materials become brittle and prone to failure at cold temperatures. Materials selection for cryogenic applications must consider thermal expansion, thermal conductivity, impact resistance, and other low temperature mechanical properties.
Biological Effects
Heat can have significant impacts on biological systems like enzymes, cells, and tissues in living organisms. Enzymes are protein molecules that catalyze chemical reactions in cells. Most enzymes have an optimal temperature range where their catalytic activity peaks. Exposing enzymes, cells or organisms to temperatures above or below this optimal range can greatly reduce enzyme activity and disrupt normal cellular processes. High temperatures can irreversibly denature and deactivate enzymes by altering their three-dimensional shape.
At the tissue and organismal level, heat impacts physiological processes like metabolism, circulation and neural function. Human body temperature is finely regulated around 37°C. Significant variations above or below this, such as in heat stroke or hypothermia, can be fatal if not treated promptly. Many biological mechanisms have evolved to help organisms adapt to different thermal environments. For example, perspiration and panting help dissipate heat, while shivering generates warmth through muscle contractions.
The effects of heat on living systems have important applications in fields like food safety, medicine and research. Heating food is used to kill harmful pathogens. Controlled heating can pasteurize and sterilize medical instruments. Studying the thermal stability of cells and molecules is critical in many biology experiments.
Weather and Climate
Heat from the sun drives convection cycles that impact weather and climate on a global scale. For example, unequal heating of the Earth’s surface creates temperature gradients that make the air unstable. This causes the hot air to rise, cool, and sink, creating circulation patterns.
On a local scale, the temperature difference between the land and sea generates sea breezes near coastlines. The land heats up faster than the water during the day, causing the warm air over land to rise. The rising air creates an area of lower pressure that draws in the relatively cool air from over the sea. At night, the opposite effect occurs, as the water retains heat longer, leading to land breezes.
The heat energy that powers thunderstorms and tornadoes comes from the sun heating the Earth’s surface. This warms and moistens the lower atmosphere creating an unstable temperature profile. As the warm air rises rapidly, thunderstorm clouds form, sometimes spawning tornadoes.
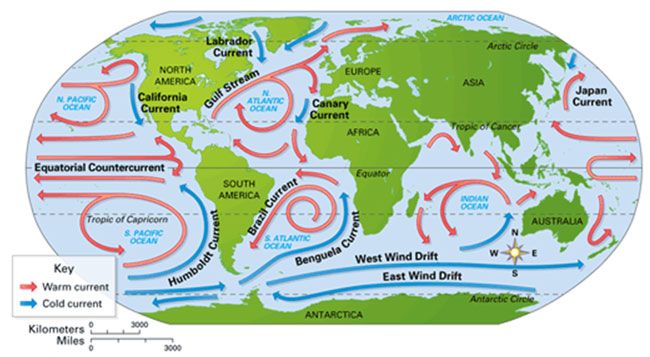
On a global scale, heat from the equator drives ocean currents like the Gulf Stream. The oceans absorb a significant amount of solar radiation near the tropics. This creates a surplus of warm water that flows north transferring heat to the cooler higher latitudes. Without this heat transport, parts of northern Europe would be much colder.
Energy Transfer
Heat naturally flows from objects at higher temperatures to objects at lower temperatures. This is described by the Second Law of Thermodynamics, which states that the entropy (disorder) of the universe always increases over time as energy disperses and differences in temperature, pressure, and chemical potential equalize.
The transfer of thermal energy from one system to another due to a temperature difference is essential for many energy technologies. Heat engines like car engines and power plants use temperature differences to generate mechanical work. Refrigerators, air conditioners, and heat pumps use work to move heat from a colder to a warmer area, transferring thermal energy against the natural flow of heat.
The efficiency of heat engines is limited by thermodynamics. Even the most efficient engines and energy technologies cannot convert all the heat they receive into useful work. Some waste heat will always be produced according to the Second Law of Thermodynamics.
Everyday Examples
Heat affects matter in many ways that we encounter in our daily lives. Here are some common examples:
Cooking: Applying heat is essential for cooking food. It causes chemical changes that alter texture, flavor, aroma, and more. For example, heat denatures proteins in meat and causes starch to gelatinize in rice or pasta. It also caramelizes sugars and drives maillard reactions for browning. Cooking makes food safer and more digestible.
Ironing: Ironing works by applying heat to clothing. The heat causes the fibers to become more pliable, allowing wrinkles to be smoothed out. Steam irons use boiling water to provide higher heat needed for thick fabrics like cotton.
Candle flames: The wax in a candle melts with heat and is drawn up the wick via capillary action. The wax vaporizes at the flame and undergoes combustion reactions with oxygen to produce light, heat, water vapor, and carbon dioxide.
As we go about our daily routines, heat is constantly acting upon the matter around us, often in subtle ways we take for granted. Paying closer attention reveals the many impacts and applications of thermal energy in everyday life.
Conclusion
In conclusion, heat affects matter in many significant ways. The most notable effects include:
- Thermal expansion – heating causes materials to expand as the motion of their molecules increases.
- Changes of state – heat provides energy for phase changes between solid, liquid, and gas states.
- Influences chemical reactions – heat speeds up reaction rates by giving molecules more kinetic energy to collide and react.
- Alters material properties – heat impacts characteristics like conductivity, hardness, and strength.
- Biological impacts – heat affects metabolism, reproduction, and survival of living organisms.
- Drives weather and climate – heat transfer in the atmosphere and oceans creates weather patterns and climate zones.
- Enables energy transfer – heat flows spontaneously from warmer objects to cooler surroundings.
In summary, heat is a critical form of energy that profoundly shapes the physical world at molecular, material, biological, meteorological, and planetary scales.