How Does A Cell Convert Solar Energy To Electrical Energy?
Photosynthesis is the process by which plants use sunlight, water, and carbon dioxide to create oxygen and energy in the form of sugar. During photosynthesis, plants convert solar energy from the sun into chemical energy that is stored in the bonds of glucose molecules. This chemical energy can later be released to fuel the plant’s activities. The overall equation for photosynthesis is:
6CO2 + 6H2O + Light Energy -> C6H12O6 + 6O2
In this equation, carbon dioxide (CO2) and water (H2O) are converted into glucose (C6H12O6) and oxygen (O2) using energy from sunlight. This process takes place in chloroplasts, specialized organelles found in plant cells that contain the chlorophyll pigment.
Chloroplasts
Chloroplasts are organelles found in plant cells and eukaryotic algae that conduct photosynthesis. They contain chlorophyll, giving them their green color. The main function of chloroplasts is to absorb light energy and convert it into chemical energy through photosynthesis.
Chloroplasts have an outer and inner membrane, with an intermembrane space in between. Inside the inner membrane is an aqueous fluid called the stroma, which contains the chloroplast DNA and ribosomes along with many enzymes. Stacks of thylakoids, which are the site of the light-dependent reactions, are also located in the stroma [1].
The thylakoid membrane contains photosynthetic pigments like chlorophyll a, chlorophyll b, and carotenoids that absorb light energy. This energy is used to drive the light-dependent reactions and produce ATP and NADPH, which provide energy for the Calvin cycle reactions that follow.
Light-Dependent Reactions
The light-dependent reactions occur in the thylakoid membranes within chloroplasts. This is where chlorophyll and other pigments absorb light energy. The absorbed light excites electrons from chlorophyll a, which pass through an electron transport chain. The electron transport chain pumps protons from the stroma into the thylakoid space, creating a proton gradient. This gradient powers ATP synthase to produce ATP. The electron transport chain also results in the production of NADPH. Overall, the light-dependent reactions convert light energy into chemical energy in the form of ATP and NADPH (1). These will be used in the next stage, the Calvin cycle.
(1) https://quizlet.com/697499160/ap-biology-2022-full-review-flash-cards/
Calvin Cycle
The Calvin cycle, also known as the light-independent reactions, is the second stage of photosynthesis. This cycle is named after American biochemist Melvin Calvin who discovered it. The Calvin cycle takes the products from the light-dependent reactions (ATP and NADPH) and uses them to fix CO2 into organic sugars like glucose. There are three key steps in the Calvin cycle: carbon fixation, reduction, and regeneration of RuBP.
Carbon fixation is the most important step, where CO2 from the atmosphere is “fixed” into an organic molecule. This reaction is catalyzed by the enzyme RuBisCO, which binds CO2 to a 5-carbon sugar called ribulose bisphosphate (RuBP). This forms an unstable 6-carbon intermediate that immediately splits into two 3-carbon molecules called 3-phosphoglycerate.
Next, the 3-phosphoglycerate molecules are reduced using electrons supplied by NADPH. This produces glyceraldehyde-3-phosphate (G3P), a 3-carbon sugar that can be used to build glucose and other carbohydrates.
Finally, most of the G3P exits the cycle to be used by the cell, while a few molecules are kept behind to regenerate RuBP. This allows the cycle to prepare for more CO2 to be fixed. ATP is used for phosphorylation reactions during this regeneration phase.
In summary, the Calvin cycle uses the energy carriers from the light reactions to fix atmospheric CO2 into organic sugars like glucose. This provides food for plants and energy for the rest of the food web.
Chemiosmosis
Chemiosmosis is the process that allows chloroplasts to produce ATP. As light energy is captured during the light-dependent reactions, it is used to pump hydrogen ions (protons) across the thylakoid membrane, creating an electrochemical gradient. This gradient builds up a high concentration of protons in the thylakoid space compared to the stroma. The protons then flow down this concentration gradient through special channels in ATP synthase enzymes. The energy from the moving protons is used by the ATP synthase to phosphorylate ADP, producing ATP 1.
The electrochemical proton gradient and ATP synthase in chloroplasts function similarly to how they operate in the inner mitochondrial membrane. The proton motive force created by the gradient allows for the large-scale production of ATP that powers cellular processes. Without chemiosmosis, photosynthesis would not be able to provide the necessary energy for plants.
Transport of Glucose
The glucose produced during photosynthesis needs to be transported out of the chloroplast so it can be used by the plant cell. This occurs through membrane proteins that act as transporters. The glucose transporter in the chloroplast membrane is powered by the proton gradient created across the thylakoid membrane. This transporter protein enables glucose to move down its concentration gradient, from inside the stroma where it is produced to outside the chloroplast where it is needed. The end result is that glucose exits the chloroplast and becomes available for other cellular processes. This transport step is essential for plants to utilize the glucose made during photosynthesis.
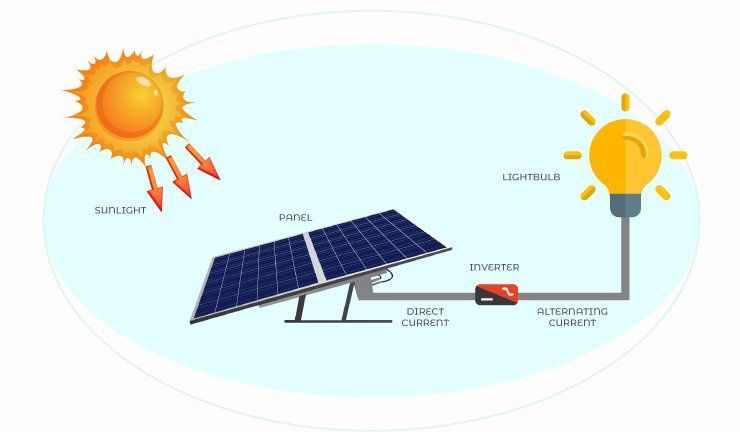
Solar Cells
Solar cells convert sunlight into electricity using the photovoltaic effect. They are made of semiconducting materials like silicon that have a specific crystalline structure, often containing impurities to create an imbalance between positive and negative charge carriers (How Do Solar Panels Work?, n.d.). When sunlight strikes the solar cell, the photons are absorbed by the semiconductor material, providing energy to electrons and promoting them into the conduction band, leaving behind positively charged holes. This generates electron-hole pairs that become separated by the built-in electric field at a p-n junction in the semiconductor (How a Solar Cell Works, n.d.).
The p-n junction is formed by doping adjacent semiconductor regions with impurities – adding extra electrons to create an n-type region, and removing electrons to generate a p-type region. The imbalance in charge carriers sets up an electric field at the junction. When light generates electron-hole pairs, the electric field separates the electrons and holes, causing electrons to accumulate on the n-side and holes on the p-side. Metal contacts attached to the n- and p-regions allow for the electrons to flow through an external circuit, generating electricity (How a Solar Cell Works, n.d.).
Thermodynamic Efficiency
When comparing the thermodynamic efficiency of natural photosynthesis in plants versus artificial photosynthesis in solar cells, studies have found that solar cells tend to be more efficient at converting sunlight into energy. One analysis from Scientific American found that the maximum efficiency of photosynthesis in plants is between 3-6%, whereas commercial solar panels typically have efficiencies of 15% or higher (Scientific American). The primary reason cited for solar cells’ higher efficiency is that they are designed solely for the purpose of converting sunlight to electricity, whereas plants evolved many other functions besides photosynthesis, which introduces inefficiencies. Researchers from Michigan State University note that plants lose a lot of energy due to evolutionary legacies that aren’t necessary in artificial systems designed purely for power conversion (MSU). So while photosynthesis is an elegant natural process, human-engineered solar cells can surpass it in efficiency by focusing only on sunlight capture and energy conversion.
Applications
Artificial photosynthesis has promising applications for improving solar cell efficiency and producing renewable hydrogen fuel (Source: https://www.mdpi.com/2313-7673/8/3/298). By mimicking natural photosynthesis, artificial systems aim to efficiently convert sunlight into storable chemical energy. This has uses for solar energy storage and on-demand fuel production.
Compared to standard solar panels, artificial photosynthesis systems can potentially aid in large-scale energy storage. They separate the light absorption and fuel generation steps, allowing the fuel to be stored for later use when needed (Source: https://www.altenergymag.com/article/2021/04/artificial-photosynthesis-as-a-renewable-energy-source/34878). This provides a storable, transportable energy source.
By improving efficiency of converting sunlight into chemical bonds, artificial photosynthesis aims to produce renewable hydrogen fuel. This hydrogen fuel could then power hydrogen fuel cell vehicles or other applications. Artificial photosynthesis provides a pathway for using sunlight to create storable solar fuels.
Conclusion
In summary, a cell converts solar energy to electrical energy through a complex process that takes place in chloroplasts containing chlorophyll. Light energy is absorbed by chlorophyll and converted into chemical energy through light-dependent reactions. Energy carriers like ATP and NADPH are generated and used in the Calvin cycle to fix carbon dioxide into sugar molecules. An electrochemical gradient is established across the thylakoid membrane during these reactions, which drives the synthesis of ATP in a process called chemiosmosis.
The sugar molecules produced can then be transported out of the chloroplast and used by the cell for energy. Though limited by thermodynamic efficiency, this elegant process of plants and algae converting sunlight into energy can be replicated artificially through solar cells and devices that mimic photosynthesis. Significant progress has been made in the field of artificial photosynthesis, though challenges remain in improving efficiency and scaling up devices.
With further advancements in nanotechnology, catalysts, and membranes, artificial systems may one day play a major role in renewable energy production. By learning from natural photosynthesis, we can work towards building a sustainable energy future.