How Do You Find The Kinetic Energy Of An Element?
Kinetic energy is the energy an object possesses due to its motion. It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having kinetic energy implies that the body has the ability to do work.
In the physical world, objects and systems interacting with one another are in constant motion. Whether a ball rolling down a hill, a car traveling down the freeway, or a gust of wind blowing leaves off of trees, kinetic energy is at play all around us. Being able to calculate an object’s kinetic energy allows us to quantify and analyze this motion.
Understanding kinetic energy is crucial in many scientific and engineering fields. Calculating kinetic energy helps us predict the future state of objects based on their current state of motion. This has important implications in studying vehicle safety, athletic performance, and energy systems. Kinetic energy is a fundamental concept with broad relevance across disciplines.
Mass in the Kinetic Energy Equation
In the kinetic energy equation, mass refers to the quantity of matter that makes up an object. More specifically, mass represents the amount of inertia or resistance to acceleration that an object possesses. This property of mass is important for kinetic energy because it determines how much energy is required to get an object moving.
An object’s mass has a direct relationship with its kinetic energy. For a given velocity, an increase in mass leads to a proportional increase in kinetic energy. This makes intuitive sense – it takes more effort and energy to accelerate a large mass to a certain speed compared to accelerating a small mass to the same speed. The more massive the object, the greater its resistance to speeding up. This resistance requires more kinetic energy to overcome.
In summary, mass is a key factor in the kinetic energy equation because it represents an object’s inertia. The more massive an object is, the greater its kinetic energy will be at a given velocity. This demonstrates the direct relationship between mass and kinetic energy.
Velocity in the Kinetic Energy Equation
In the kinetic energy equation, velocity refers to the speed of an object in a particular direction. An object’s velocity is a vector quantity that expresses both the object’s speed (or rate of motion) and the direction of its movement.
Velocity plays an important role in determining an object’s kinetic energy. Kinetic energy increases proportionally to the square of an object’s velocity. This means that if an object’s velocity doubles, its kinetic energy will increase by a factor of four. And if the velocity triples, the kinetic energy will increase by a factor of nine, and so on.
So an object moving at a higher velocity, like a bullet speeding out of a gun, has much greater kinetic energy than an object moving slowly, like a snail. This is because the faster object’s energy is amplified exponentially based on its high velocity squared. Understanding this relationship allows physicists to calculate the kinetic energy for objects moving at different known velocities.
The Kinetic Energy Equation
The kinetic energy (KE) of an object can be calculated using the following equation:
KE = 0.5 x m x v2
Where:
- m = mass of the object (in kilograms)
- v = velocity of the object (in meters per second)
This is known as the kinetic energy equation. It shows that the kinetic energy of an object depends on two variables – its mass and its velocity. The faster an object moves, the more kinetic energy it possesses. Likewise, the more massive an object is, the greater its kinetic energy will be at a given velocity.
Let’s break down each component of the kinetic energy equation:
- 0.5 is a constant that is derived from the physics of kinetic energy. It simplifies the equation.
- m refers to the object’s mass, measured in kilograms. The greater the mass, the greater the kinetic energy.
- v refers to the object’s velocity, measured in meters per second. Velocity is a vector quantity that denotes speed and direction.
- v2 squares the velocity, which exponentially scales the kinetic energy. Even a small change in velocity results in a large change in kinetic energy.
By plugging in values for mass and velocity, you can use this equation to find the kinetic energy of any moving object. The units will be in joules (J), the SI unit for energy.
Calculating Kinetic Energy
Kinetic energy can be calculated using the following equation:
KE = 0.5 x m x v2
Where:
- KE is kinetic energy in joules (J)
- m is mass in kilograms (kg)
- v is velocity in meters per second (m/s)
Let’s look at some examples of using this equation to find kinetic energy:
– A 2 kg object moving at 4 m/s has a kinetic energy of:
KE = 0.5 x 2 kg x (4 m/s)2 = 16 J
– A 5 kg object moving at 3 m/s has a kinetic energy of:
KE = 0.5 x 5 kg x (3 m/s)2 = 22.5 J
As seen in these examples, kinetic energy is expressed in units of joules (J) or kg•m2/s2. The faster an object moves, the greater its kinetic energy since velocity is squared in the kinetic energy equation.
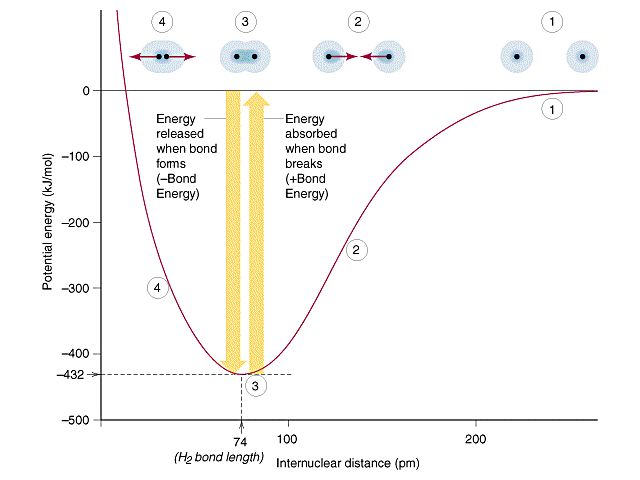
Kinetic Energy of Atoms and Molecules
At the atomic and molecular scale, kinetic energy arises from the motion of atoms and molecules. As with macroscopic objects, the kinetic energy of an atom or molecule depends on its mass and velocity.
However, at the atomic scale things get a bit more complex. Atoms and molecules are constantly vibrating and rotating, and these vibrational and rotational motions contribute to their kinetic energies. The total kinetic energy of an atom or molecule is the sum of the kinetic energy from vibrational motion, rotational motion, and translational motion of the molecule as a whole.
For individual atoms, the kinetic energy comes primarily from translational motion as they move about randomly. Heavier atoms move more slowly so they have lower average kinetic energy than lighter atoms at the same temperature.
For molecules, kinetic energy has additional contributions from vibrational and rotational motions. Vibrational kinetic energy depends on the bonds between the atoms in the molecule. The stronger the bonds, the higher the vibrational frequency and energy. Rotational kinetic energy depends on the moments of inertia of the molecule.
In both atoms and molecules, temperature is a measure of average kinetic energy. Higher temperatures mean greater atomic/molecular motion and higher average kinetic energy. When a substance is heated, the atoms/molecules vibrate and rotate more vigorously, increasing their kinetic energies.
Understanding kinetic energy at the atomic scale is important for modeling chemical reactions, thermodynamics, heat transfer, and other phenomena. The distribution of kinetic energies of atoms and molecules determines the rates and mechanisms of chemical reactions as well as macroscopic thermal properties.
Methods for Measuring Kinetic Energy
There are several experimental methods that can be used to measure the kinetic energy of atoms and molecules. Some of the most common methods include:
Calorimetry
Calorimetry involves measuring the heat absorbed or released during a chemical reaction. Since heat and temperature are related to kinetic energy at the molecular level, calorimetry provides an indirect measurement of kinetic energy.
Spectroscopy
Techniques like infrared spectroscopy and Raman spectroscopy analyze the frequencies of light absorbed or emitted during transitions between molecular energy levels. Since these energy levels correspond to different kinetic energies of the molecules, spectroscopy enables the measurement of kinetic energy.
Collisions
The kinetic energy of atoms and molecules can also be measured by allowing them to collide with other particles and measuring the energy transfers that occur. Examples include collision-induced dissociation experiments and crossed molecular beam methods.
Time-of-Flight Mass Spectrometry
In time-of-flight mass spectrometry, ions are accelerated through an electric field and their velocities are measured. Since kinetic energy is related to velocity, this enables the determination of kinetic energies.
By utilizing these and other experimental techniques, scientists can quantify the kinetic energies of atoms, molecules, and chemical systems under different conditions and gain insights into chemical and physical processes at the molecular level.
Applications and Examples
Kinetic energy has many practical applications in the real world. Here are some examples of calculating kinetic energy in different contexts:
Physics
In physics, kinetic energy is used to analyze collisions between objects. For example, you can calculate the kinetic energy of a bowling ball right before and after it collides with pins to understand energy transfer during collisions.
Engineering
Engineers may calculate kinetic energy to design structures and vehicles that can withstand impacts. For example, the kinetic energy of a moving car needs to be considered when designing highways and guardrails.
Chemistry
In chemistry, the kinetic energy of atoms and molecules determines reaction rates and phase changes. Higher kinetic energy means atoms and molecules move faster and are more likely to react.
Sports Science
In sports, kinetic energy is used to analyze athletic performance. The kinetic energy of a sprinter can be calculated from their mass and running velocity.
Meteorology
Meteorologists may calculate the kinetic energy of wind patterns and air masses to predict storm severity and wind damage.
Overall, kinetic energy has broad relevance across many scientific and engineering fields. Calculating kinetic energy provides insight into system dynamics and processes in a wide range of real-world applications.
Common Misconceptions
There are a few common misconceptions people have about kinetic energy:
Kinetic energy depends on velocity, not speed – One of the most common mistakes is thinking that kinetic energy depends only on the speed of an object, when in fact it depends on the velocity. Speed refers only to the magnitude of velocity, whereas velocity accounts for both speed and direction. Kinetic energy depends on the square of velocity, so the direction matters.
Greater mass always means greater kinetic energy – While kinetic energy does depend on mass, it also depends on the square of velocity. So a small mass moving very fast can have greater kinetic energy than a large mass moving slowly. Both factors need to be considered.
Kinetic energy is the same as momentum – Momentum accounts for mass and velocity, but does not square the velocity like kinetic energy does. So two objects can have the same momentum but different kinetic energies depending on their velocity.
Kinetic energy only applies to moving objects – While kinetic energy depends on motion, all matter has kinetic energy down to the atomic level due to the motion of atoms and molecules. Even stationary objects have internal kinetic energy.
Understanding these common misconceptions can help clarify how to properly calculate and apply the concepts around kinetic energy. Keeping in mind how both mass and the square of velocity factor into the kinetic energy equation is key.
Conclusion
In summary, kinetic energy is the energy that an object or particle has due to its motion. It depends on two key variables – the mass of the object and its velocity. The kinetic energy increases exponentially as the velocity increases linearly. This is represented by the kinetic energy equation: Kinetic Energy = (1/2) x Mass x Velocity^2.
Understanding kinetic energy is crucial across many scientific fields, especially physics and chemistry. It allows us to calculate the energy stored in moving objects, predict collisions, and model molecular interactions. Kinetic energy concepts are applied in mechanical engineering, ballistics, space exploration, and more. By mastering the simple kinetic energy equation and how to apply it, we gain profound insight into the motion of objects large and small.
In conclusion, kinetic energy provides a mathematical framework for analyzing motion and is a fundamental concept in physics. This overview summarized the key variables, equations, and applications – providing a solid basis for utilizing kinetic energy calculations.