How Do Electrons Move?
Electrons are tiny particles of matter that carry a negative electric charge. They are found outside the nucleus (the dense core of an atom containing protons and neutrons) of every atom and move around it. The movement of electrons is the driving force behind many applications we use every day including electricity, batteries and electronics.
Understanding electron movement is essential to harnessing electrical energy. Flowing electrons in a conductor like a wire creates an electric current that powers devices and appliances in our homes and offices. The controlled movement of electrons enables modern technology like computers, cell phones, electric motors and more.
On a microscopic scale, electrons behave like both particles and waves. Their motion is governed by principles of quantum mechanics. Electrons can jump between designated regions around the nucleus called electron shells and subshells. The exact path of an individual electron is probabilistic, but in aggregate, electrons flow in predictable patterns.
This overview will examine how electrons move through atoms, solids, circuits and devices. Gaining a better understanding of electron behavior helps explain the properties of different materials, charge flow, and the many technological applications that depend on electron movement.
Electrons and Electric Charge
Electrons possess a negative electric charge. Electric charge refers to a fundamental property of matter that causes particles to experience a force when placed in an electromagnetic field. There are two types of electric charge – positive and negative. Positively charged particles repel other positively charged particles but attract negatively charged particles. Similarly, negatively charged particles repel other negatively charged particles but attract positively charged particles.
The electron has a negative charge, meaning it repels other electrons due to their like charges. The amount of charge on a single electron is equal to -1.602176634 x 10-19 coulombs. This is considered the elementary charge and is used as the standard unit of charge for particles. The reason electrons have a negative charge relates to the structure of atoms. Atoms contain subatomic particles like electrons, protons, and neutrons. Protons have a positive charge while electrons have a negative charge. The charge on a neutral atom balances out so that the number of protons equals the number of electrons.
When an atom gains or loses electrons, it develops a net electric charge and becomes an ion. The gain or loss of electrons during interactions between atoms is key for phenomena like chemical reactions and the flow of electricity. Overall, the negative charge carried by electrons is a fundamental property that governs their behavior within atoms, molecules, circuits, and more.
Electron Shells and Orbital Models
Electrons occupy distinct electron shells and orbitals around the nucleus of an atom. The electron shells represent different energy levels that electrons can occupy. Each shell can hold a maximum number of electrons – the first shell holds up to 2 electrons, the second up to 8, the third up to 18, and so on. The shells fill from the inside out, with the outermost shell being called the valence shell.
Within each electron shell are subshells and orbitals that further describe the location and properties of electrons. Several key atomic models have been proposed to explain how electrons are arranged in these shells and orbitals:
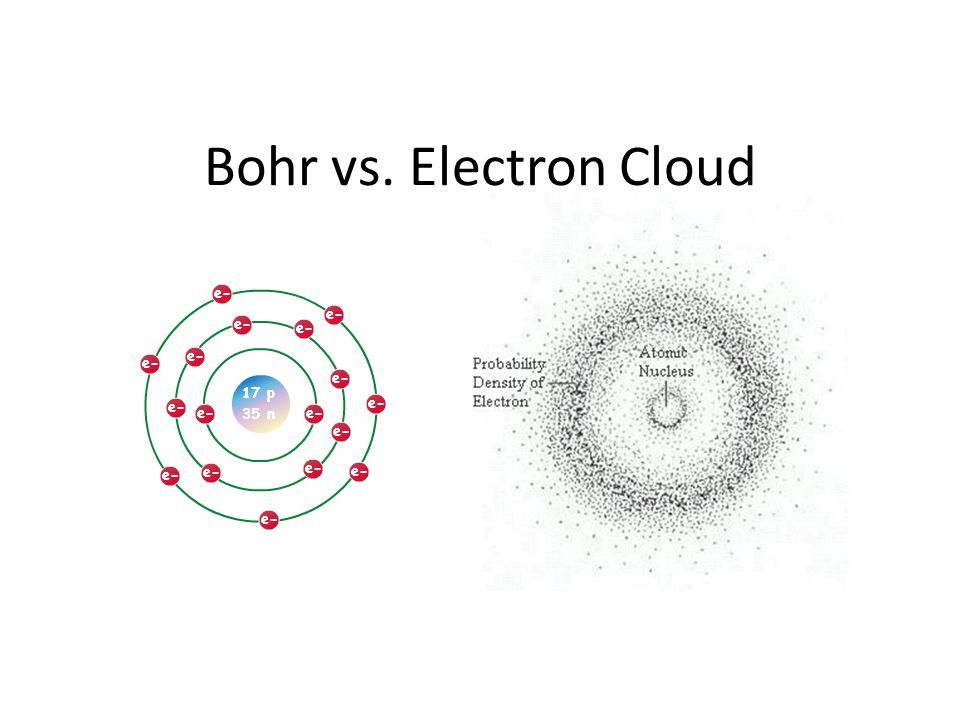
- Bohr Model – A simplified model that depicts electron shells as concentric circles around the nucleus. It describes electrons traveling in circular orbits, but does not account for subshells. Accurately predicted the ordering of shells.
- Quantum Mechanical Model – A more complex model based on quantum physics and Schrödinger’s wave equation. Describes probability clouds and spatial orbitals (s, p, d, f) within shells, more accurately predicting electron behavior.
- Aufbau Principle – Describes the filling order of electron shells/orbitals – lower energy levels fill first in order of increasing atomic number.
These orbital models explain periodic trends in the elements, electron transitions that produce light, and the shape of molecular orbitals in chemical bonds.
Quantum Physics and Electron Behavior
Quantum physics emerged in the early 20th century as scientists probed deeper into the subatomic world. They found that the classical physics laws that governed objects and forces at the macro level of matter did not apply as they ventured into the nanoscale realm of atoms and subatomic particles.
Instead of physical orbits, electrons occupy fuzzy clouds of probable locations around the atom’s nucleus. These clouds represent the likeliest positions an electron might occupy. But due to the wave-particle duality of matter at the quantum scale, the electron does not have a precise position until it is measured – instead existing as a superposition of all the probabilities within its orbital. Thus quantum physics tells us that we can never know for certain where an electron is located within an atom’s electron cloud until we interact with it to observe and measure its actual position.
However, we can map the geometry of the most probable locations of electrons within their orbitals, which do follow distinct electron shell patterns in most atoms. The s,p,d and f orbitals have differing 3D models that provide an helpful visualization of where electrons are most probably found orbiting the nucleus. But the fuzziness and unpredictability of matter on the nanoscale is counterintuitive to everyday human experience, so quantum physics helps explain the probabilistic realm that regulates electrons’ actual locations, energy levels and movements within atoms and molecules.
Electric Current and Electron Flow
Electric current is the flow of electric charges, usually electrons in metal wires or other conductors. Metals have loosely bound valence electrons which can move more freely through the metal. When a voltage difference is applied to ends of a conductor, it applies a force on negatively charged electrons, causing them to move from a region of higher potential to lower potential energy. This flow and motion of electrons is called an electric current.
The movement of electrons in a wire or electrical conductor constitutes a net flow of negative charge. By convention, the direction of electric current is taken as the direction of motion of positive charge. So in an electrical circuit where negatively charged electrons are flowing, the electric current will be in the opposite direction of actual electron motion.
An electric current will flow when a closed conducting loop contains a voltage source. The voltage source, such as a battery, provides energy to accelerate electrons through the circuit, overcoming resistance in the wires. The generated electric current results from the organized motion of electrons as they travel through conductors.
Conductors, Insulators, and Semiconductors
The ability of electrons to move freely is what enables conductivity in various materials. Conductors contain a significant number of free electrons that can carry electric currents. Metals like copper and aluminum are common conductors used in electrical wiring.
Insulators, on the other hand, have very few free electrons. Electrons in insulators are tightly bound to their atoms and atomic structure, which prevents free electron flow and makes them non-conductive. Most plastics, glass, wood, and air are effective insulators.
Semiconductors fall somewhere inbetween conductors and insulators. They possess moderate electron conductivity and their conductivity also varies based on temperature, light exposure, and doping (the deliberate introduction of impurities). Silicon and germanium are examples of semiconductor materials that enabled modern electronic devices.
Electron Movement in Batteries/Cells
Batteries or galvanic cells utilize chemical reactions to generate electricity through the movement of electrons between a reductant (provides electrons) and an oxidant (accepts electrons). The battery consists of a positive electrode (cathode) and a negative electrode (anode), typically made of different materials, separated by an electrolyte solution.
Oxidation occurs at the anode, where electrons are “lost” by the reductant material. This generates surplus electrons that move towards the cathode. Meanwhile the oxidant at the cathode gains electrons from the anode, undergoing reduction. Electrons flow from the anode to the cathode through the connected circuit, generating an electric current that powers the device.
Common battery chemistries utilize metal electrodes and salt solutions for oxidation and reduction reactions. For example, alkaline batteries use zinc as the anode (provides electrons) and manganese dioxide as the cathode (accepts electrons), with an alkaline electrolyte facilitating ion flow to maintain overall charge neutrality.
As the battery discharges over time, the reductant oxidizes, producing electrons, while the oxidant gets reduced by accepting those electrons. This continues until the fully oxidized/reduced states are reached and the battery runs out. The movement of electrons from anode to cathode via chemical reactions is thus central to battery operation.
Photoelectric Effect
The photoelectric effect refers to the phenomenon where electrons are emitted from the surface of a material when light shines on it. It was first discovered experimentally by Heinrich Hertz in 1887. Later, Albert Einstein provided the correct explanation for it in 1905, and it was an important step in the development of quantum theory.
When light shines on a metal surface, electrons can absorb the energy from photons in the light and get excited to higher energy levels. If the photon energy is high enough, some of these electrons can gain enough energy to break free completely from the metal and leave its surface. Even if the light shines for a very brief time, some electrons emit immediately.
The effect of the light on electron emission depends on both the intensity and the wavelength (frequency) of the light. Increasing the intensity gives more photons hitting the metal per second, resulting in more electrons emitted. But the wavelength also matters because each photon carries a specific amount of energy that depends on its frequency. A higher frequency photon has higher energy. So light above a threshold frequency is required to emit any electrons, no matter how intense a lower frequency light is made. This demonstrates the particle-like quantum nature of light.
Overall, the photoelectric effect was crucial in developing the modern quantum mechanical understanding of light and electrons. Einstein explained it by treating light as a particle (photons), an idea that was contrary to the classical wave theory of light but turned out to be correct. The effect shows light can transfer discrete amounts of energy to electrons in metals, causing electron emission.
Cathode Ray Tubes
Cathode ray tubes (CRTs) are vacuum tubes that use beams of electrons to display images. Inside a CRT, one or more electron guns emit narrow beams of electrons. These beams are focused and accelerated towards the screen (phosphor-coated inner surface) at the front end of the tube. When the fast-moving electrons strike the phosphor screen, it produces areas of visible light through cathodoluminescence.
CRTs were commonly used in older television sets and computer monitors. In cathode ray tube televisions, one electron gun was used to produce a single beam that rapidly moved back and forth across the screen by magnetic deflection, progressively “painting” the screen line by line to form the images. In color CRT TVs and monitors, three electron guns and beams (one for each primary color) scanned the screen in synchronization. By controlling the intensity of the electron beams and how long they impacted each phosphor dot, the right mix of colors and brightness could be produced to display any color image.
CRT displays were the dominant display technology for TVs and computer monitors during the latter half of the 20th century, but they were large in size, required high voltages, and had other limitations in performance and safety. As display technologies improved, CRT TVs and monitors were eventually replaced by flat-panel displays like LCD, LED, and OLED screens which were thinner, lighter, more energy efficient, and had better resolution and overall picture quality.
Electron Applications and Impacts
Understanding and controlling the movement of electrons has enabled numerous key technologies and applications used globally. Electron flow enables almost all electronics, from small sensors to massive computational devices. By directing electrons through semi-conducting materials using transistors and integrated circuits, humankind has been able to build computers, phones, and a globally-connected internet.
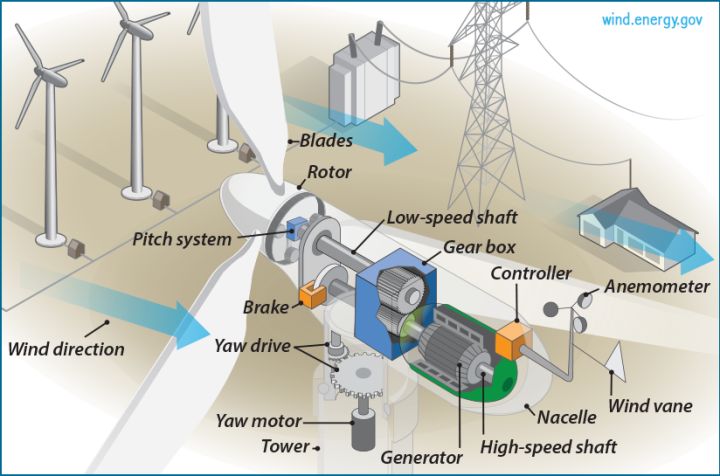
Electricity generation and transmission relies on electron movement between atoms to transport energy over distances. Coal, gas, hydroelectric, solar, and wind power plants all utilize rotational generators that produce electricity by moving electrons using magnetic fields.
Additionally, electron flow powers motors that drive machinery of all sizes and applications, from tiny hard disk drive motors spinning at 10,000 RPM to enormous industrial motors capable of turning gears weighing tons. Electron movement enables refrigeration, ventilation, lighting, heating, and cooling systems that impact quality of life.
Photons striking metals and releasing electrons (the photoelectric effect) allows for technologies like cameras, TVs, and solar panels. The studied understanding of electrons has also enabled advanced scientific instruments like electron microscopes, which can magnify objects to nearly the atomic level.