How Are Living Organisms Part Of The Cycling Process?
Cycling processes are essential biochemical pathways that recycle and reuse elements and nutrients within ecosystems. These cycles allow elements like carbon, nitrogen, and phosphorus to move continuously through the biotic (living) and abiotic (nonliving) components of an environment.
Nutrient cycling enables the repeated use of finite resources, promoting sustainability within natural systems. It replenishes resources that living organisms need to survive, like carbon for constructing organic compounds and nitrogen for building proteins. Without the recycling of these chemical elements, ecosystems would rapidly deplete the available resources and become unable to support life.
Cycling processes create interdependency between organisms. The waste products from one organism become food for another. This closed-loop system allows maximum utilization of nutrients within a habitat. Proper cycling provides environmental stability and enables the continuous exchange of energy and matter necessary to sustain biodiversity.
Photosynthesis
Photosynthesis is the process that plants and some bacteria use to convert carbon dioxide and water into glucose and oxygen using energy from sunlight. This process is essential for cycling carbon in the biosphere.
During photosynthesis, plants absorb carbon dioxide (CO2) from the atmosphere through their leaves. The CO2 molecules combine with water molecules (H2O) and, using energy from sunlight, are converted into glucose (C6H12O6) and oxygen (O2). The glucose provides food and energy for the plants, while the oxygen is released back into the atmosphere as a waste product.
Photosynthesis cycles carbon through the biosphere by removing CO2 from the atmosphere and converting it into organic carbon compounds like glucose. This organic carbon then flows through food webs as organisms consume plants or other organisms that ate plants. The carbon returns to the atmosphere as CO2 through the respiration of heterotrophs that feed on the plants.
Without photosynthesis, carbon dioxide would accumulate in the atmosphere and oxygen levels would decline. By continuously converting CO2 into O2, photosynthesis maintains breathable air and balances CO2 levels in the atmosphere. In this way, photosynthesis drives the global carbon cycle and enables a habitable planet.
Cellular Respiration
Cellular respiration is the process by which organisms like plants, animals, and bacteria convert nutrients into energy for cell functions like growth and reproduction. It plays a key role in oxygen cycling on Earth.
During cellular respiration, glucose and oxygen are converted into carbon dioxide, water, and energy. The overall chemical reaction looks like this:
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + Energy (ATP)
This reaction uses oxygen from the atmosphere while releasing carbon dioxide back into the air. Through this process, oxygen is continuously cycled between organisms and the environment. Plants and phytoplankton take in CO2 and release O2 during photosynthesis, while animals, bacteria, fungi and other respiring organisms use O2 and give off CO2.
Cellular respiration is essential for generating energy that powers life processes. The cycling of oxygen between photosynthetic and respiring organisms helps maintain balanced atmospheric oxygen levels necessary to sustain life on Earth.
Nitrogen Fixation
Nitrogen fixation is a process where nitrogen gas (N2) in the atmosphere is converted into a usable form of nitrogen like ammonia (NH3). This process is critical for life because plants cannot use nitrogen gas directly. Nitrogen fixation makes nitrogen available in the soil so it can be utilized by plants.
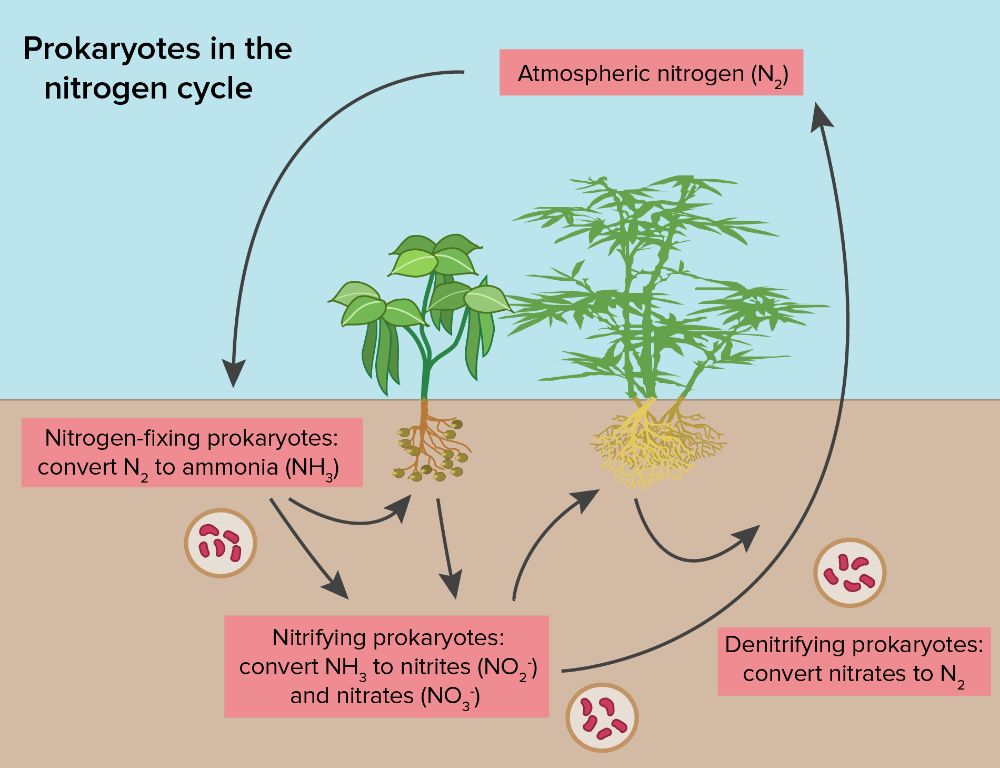
Nitrogen fixation is primarily carried out by specialized bacteria that live symbiotically in the roots of legumes like beans, peas, and soybeans. The bacteria are able to convert nitrogen gas into ammonia through bacterial enzymes called nitrogenases. The ammonia produced can then be used by plants to synthesize proteins and nucleic acids.
Free-living nitrogen fixing bacteria also live in soils and wetlands. Cyanobacteria, a type of bacteria that conducts photosynthesis, are able to fix nitrogen in aquatic environments. Lightning strikes also result in small amounts of nitrogen fixation by providing enough energy to convert nitrogen in the air into nitrate, which plants can use.
Without nitrogen fixation, life would not be possible. It transforms inert nitrogen gas into reactive nitrogen that living organisms need. Nitrogen fixation replenishes the nitrogen lost through denitrification and leaching, closing the nitrogen cycle.
Ammonification
Ammonification is an important process in the nitrogen cycle where organic nitrogen is converted to ammonium (NH4+). This process is carried out by decomposers such as fungi and bacteria that break down organic matter containing nitrogen, releasing ammonium as a metabolic byproduct.
During ammonification, proteins and nucleic acids in decaying organic matter are broken down. Decomposers secrete enzymes that hydrolyze these compounds, converting their nitrogen into ammonium. Some examples of organisms that ammonify organic matter include Bacillus, Clostridium, Pseudomonas, and many fungi.
This process replenishes the soil with ammonium that plants can readily absorb and assimilate into amino acids and proteins. Ammonification is crucial for the continued recycling of nitrogen required for plant and microbial growth. It allows organic nitrogen to re-enter the soil in bioavailable forms. Without the activity of decomposers generating ammonium, nitrogen would be sequestered in organic matter and unavailable to support new growth.
Thus, ammonification connects two important nitrogen transformations – the decomposition of organic matter and the production of ammonium that can be further oxidized and utilized by organisms. It helps close the nitrogen cycle and enables nitrogen to be reused indefinitely within an ecosystem.
Nitrification
Nitrification is an important step in the nitrogen cycle where ammonia is converted to nitrite and then to nitrate by specialized bacteria. This process occurs in soil and water environments and is critical for making nitrogen available to plants and other organisms.
There are two steps in nitrification:
- Ammonia-oxidizing bacteria convert ammonia (NH3) to nitrite (NO2-). Examples of ammonia oxidizers include species of Nitrosomonas.
- Nitrite-oxidizing bacteria then convert the nitrite into nitrate (NO3-). Common nitrite oxidizers are species of Nitrobacter.
These nitrifying bacteria get their energy for growth from the oxidation of nitrogen compounds. By converting ammonia to nitrate, they make nitrogen available to plants and other autotrophs in a form they can use. Nitrate acts as plant fertilizer. Without nitrification, much of the nitrogen in the environment would be unavailable for biological use.
Nitrification occurs naturally in many environments but can also be facilitated artificially in wastewater treatment plants, aquaria, agricultural soils and other man-made systems. Understanding and optimizing nitrification is important for maintaining healthy ecosystems and productive soils and aquaculture environments.
Denitrification
Denitrification is a critical process in the nitrogen cycle where certain bacteria convert nitrate (NO3-) into nitrogen gas (N2), which returns nitrogen to the atmosphere. This occurs in oxygen-depleted environments such as wetlands, lake sediments, groundwater, and deep ocean zones. Denitrifying bacteria, such as Pseudomonas and Clostridium, use nitrate as an alternative electron acceptor for respiration in place of oxygen. This allows the bacteria to continue respiratory metabolism and obtain energy in the absence of oxygen.
The overall reaction for denitrification is:
2NO3- + 10e- + 12H+ → N2 + 6H2O
Denitrification removes excess reactive nitrogen from ecosystems and prevents the over-enrichment of waterways, which can lead to algal blooms, dead zones, and fish kills. However, too much denitrification can also deplete essential soil nitrogen needed by plants. A delicate balance is required. Estimates indicate that denitrification returns 20-35% of fixed nitrogen back to the atmosphere.
Human activities are increasing the amount of nitrogen available for denitrification. Fertilizer use, legume cultivation, and the combustion of fossil fuels produce reactive nitrogen that makes its way into groundwater and aquatic systems. This extra nitrate allows denitrifiers to flourish, amplifying rates of denitrification. Some scientists think this may be offsetting a portion of the excess nitrogen from human sources that would otherwise accumulate in the environment.
In sum, denitrification forms a critical link in the nitrogen cycle that recycles nitrogen back to the atmosphere and prevents nitrogen overloading in ecosystems. However, enhanced denitrification due to human activities could reduce soil fertility and plant growth over time if the nitrogen losses become excessive.
Decomposition
Decomposition is a key process in the cycling of nutrients through ecosystems. When organisms die, their remains are broken down by decomposers like bacteria and fungi. These decomposers secrete enzymes that digest complex organic molecules in dead organisms into simpler inorganic compounds like carbon dioxide, water, and mineral salts.
The decomposition process returns nutrients like nitrogen, phosphorus, and potassium to the soil and makes them available for reuse by plants. As plants take up these inorganic nutrients, incorporate them into new organic compounds through photosynthesis, and pass them up the food chain, a continuous cycle of nutrient recycling occurs.
Decomposition facilitates major biogeochemical cycles. As organic carbon is decomposed and respired back into the atmosphere as carbon dioxide, this fuels the carbon cycle. Decomposition also provides nutrients that drive the nitrogen, phosphorus, and sulfur cycles. By breaking down dead biomass and regenerating nutrients from waste products, decomposers act as nature’s recyclers and sustain ecosystems.
Bioaccumulation
Bioaccumulation refers to the buildup of substances, such as pesticides, chemicals, and heavy metals, in an organism. It occurs when an organism absorbs a toxic substance at a rate faster than the substance can be metabolized or excreted. The toxin accumulates increasingly over time and up the food chain in a process called biomagnification.
Heavy metals like mercury provide a classic example of bioaccumulation. Microscopic plants such as algae absorb mercury present in water. Small animals eat lots of algae and accumulate more mercury in their tissues. Larger predators eat many small animals and build up even higher mercury concentrations. At the top of the food chain, fish like tuna or sharks can have mercury levels millions of times higher than the surrounding water.
Persistent organic pollutants (POPs) also severely bioaccumulate. These toxic chemicals are lipophilic, meaning they dissolve in fat rather than water. POPs accumulate in fatty tissues as they move up the food chain. Animals at the top of the chain end up with dangerous POP levels that can lead to neurological damage, cancers, and reproductive issues.
Bioaccumulation poses risks not just for top predators, but for entire ecosystems. But it also provides a tool for assessing environmental health. Scientists can measure toxin levels in indicator species to monitor pollution in the surrounding habitat.
Conclusion
As we have seen, living organisms play a crucial role in cycling processes that sustain life on Earth. Through key processes like photosynthesis, cellular respiration, nitrogen fixation, nitrification, and decomposition, organisms facilitate the continuous movement of nutrients like carbon, nitrogen, and phosphorus through the biosphere. Photosynthesizing plants, algae, and some bacteria take in carbon dioxide and energy from the sun to produce organic compounds and oxygen. This introduces carbon from the atmosphere into the food web. Cellular respiration, carried out by all organisms, releases carbon back into the atmosphere. Nitrogen fixation by bacteria converts inert nitrogen gas into biologically usable forms like ammonia. Further microbial processes produce nitrates that plants rely on, which are eventually converted back to nitrogen gas by denitrifying bacteria, closing the nitrogen cycle. When organisms die, decomposers break down their organic matter, returning nutrients to the soil and air. Through these interconnected processes of nutrient uptake, conversion, and release, living organisms sustain the cycling of matter that makes life possible.