How Are Energy And Work Different?
Energy and work are two fundamental concepts in physics that are closely related but have distinct differences. Energy is the ability to do work or produce heat. It exists in various forms such as kinetic energy, potential energy, thermal energy, chemical energy, nuclear energy, and more. Work on the other hand is the process of exerting a force to move an object over some distance. While energy is stored within a system, work is energy being transferred between systems or converted from one form to another.
This article will explore the nuanced differences between energy and work. We’ll define key terms, units of measurement, and real-world examples to build an intuitive understanding. You’ll learn how energy can be transferred, transformed, and conserved according to the laws of thermodynamics. We’ll also cover calculations relating work and energy to solidify these important concepts.
Forms of Energy
Energy comes in many different forms that can be categorized into two main types: potential energy and kinetic energy. Potential energy is stored energy that has the potential to do work, while kinetic energy is energy in motion. Here are some of the most common forms of energy:
Potential Energy
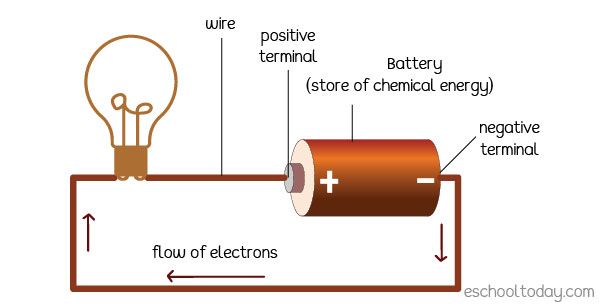
– Chemical Energy – Energy stored in the bonds between atoms and molecules. Batteries and food are examples of stored chemical energy.
– Nuclear Energy – Energy stored in the nucleus of an atom. Nuclear power plants release this energy through fission or fusion.
– Gravitational Potential Energy – Energy stored in an object’s height above ground. When the object falls, this potential energy converts into kinetic energy.
– Elastic Potential Energy – Energy stored in compressed or stretched elastic materials like springs or rubber bands.
– Electrical Potential Energy – Energy stored in an electric field. This energy can be released and converted into other forms.
Kinetic Energy
– Radiant Energy – Electromagnetic energy that travels in waves, such as visible light, ultraviolet rays, infrared rays, radio waves, etc.
– Thermal Energy – The internal energy of an object due to the motion of its atoms and molecules. Related to an object’s temperature.
– Motion Energy – The energy possessed by an object due to its motion. The faster an object moves, the more kinetic energy it has.
– Sound Energy – Caused by the back-and-forth vibration of matter, which creates sound waves that travel through mediums like air and water.
– Electrical Energy – The movement of electrons. Lightning is an example of released electrical energy.
Work Defined
Work is defined as force applied over a distance. When a force displaces an object, it does work on the object. More specifically, work occurs when an object moves in the same direction as the force acting on it. The amount of work equals the size of the force times the distance the object moves.
Work involves transferring energy from one object or system to another. For example, when you lift a heavy box from the ground, you do work on the box by applying an upward force over a vertical distance. This transfers your muscular energy into gravitational potential energy stored in the box. Without work being performed, energy cannot be transferred between objects or systems.
Units of Measurement
When measuring energy and work, it’s important to use the proper units. The standard unit of measurement for energy is the joule (J). For work, the units are joules (J) or newton-meters (N•m).
One joule is defined as the amount of work done by a force of one newton moving an object one meter. A newton is the force required to accelerate one kilogram of mass by one meter per second squared. So a joule represents the application of a one newton force through a distance of one meter.
In mechanical systems, energy and work can easily be converted back and forth using these units. For example, a 1000 J energy source can produce 1000 N•m of work. The joule and newton-meter units allow precise calculations relating force, distance, mass, and energy.
Energy Storage
Energy comes in many different forms that can be harnessed and used. Some common examples are chemical energy, thermal energy, mechanical energy, electrical energy, light energy, nuclear energy, and more. A key difference between energy and work is that energy can be stored for later use, while work happens instantaneously.
For instance, batteries store chemical energy that can be used to power flashlights, cars, phones, and more. Gasoline contains chemical energy that can propel vehicles when ignited in a combustion engine. Food has chemical energy that organisms use for growth, motion, and bodily functions. Springs and elastic bands store mechanical energy when compressed or stretched, which is released when they recoil. Water held behind a dam contains gravitational potential energy, which is converted to electricity as it flows through turbines. Nuclear fuels contain energy stored within atomic bonds that is released as heat through fission and fusion reactions.
In all these examples, energy is being stored in some form for later use. This stored energy can then be used to perform work. However, work itself is not stored – it is an instantaneous transfer of energy to move an object or generate heat. For example, when releasing the stored energy in a compressed spring, work is done to accelerate a toy car. But the work ends once the spring has recoiled and the car is in motion. The work happened in that instant, transforming the stored energy into kinetic energy.
So in summary, energy comes in many convertible forms and can be stored, while work is a momentary phenomenon that transfers energy to create motion or heat. This key distinction helps explain how energy storage enables the continuous transfer of energy through work over time.
Conservation Laws
One of the key differences between energy and work is that energy is always conserved, while work is not necessarily conserved. This understanding is based on two important laws of thermodynamics:
The First Law of Thermodynamics states that energy can neither be created nor destroyed – it can only change form. For example, chemical energy from food can change into kinetic energy as a person walks. The total energy remains constant.
In contrast, the work done by a system can appear or disappear. Work involves force moving an object over a distance. If frictional forces act opposite to the motion, the work done against friction results in heat rather than motion. The work does not remain constant.
In summary, the conservation of energy is a fundamental physical law, while work is dependent on forces and not strictly conserved. This distinction highlights a key difference between the two concepts.
Real World Examples
Energy and work are abstract concepts that are easier to understand through real world examples. Here are a couple common examples that demonstrate their differences:
Batteries Store Energy
Batteries are devices that store chemical energy and convert it into electrical energy. The energy is stored inside the battery until it is used to power something, like a flashlight. The stored energy enables the battery to do work by powering the flashlight. But the battery itself doesn’t actually do any work, it just releases its stored energy.
Falling Object Does Work
When an object falls, it gains kinetic energy. If it falls onto a surface, it can do work by applying a force over a distance to the surface. For example, if a hammer falls onto a nail, it does work on the nail by driving it into a piece of wood. The hammer had potential energy when it was held up high, which turned into kinetic energy as it fell. This kinetic energy allowed it to do work on the nail.
Energy Transformation
One of the most important principles to understand is that energy can change form. For example, when you turn on a light bulb, electrical energy is transformed into light and heat energy. Even though energy changes form, the total amount of energy in the universe remains constant. This is known as the law of conservation of energy.
Work and energy are closely related. In physics, work is defined as force applied over a distance. When work is done, energy is transferred from one object to another or transformed from one form to another. For example, when you lift a heavy box to a high shelf, you do work by applying force over a vertical distance. This work causes energy to transfer from your muscles to the box in the form of gravitational potential energy.
In order for work to occur, there must be energy available to transfer. Devices like engines and machines are designed to convert energy into useful work. For instance, a wind turbine converts the kinetic energy of wind into electrical energy through a generator. Understanding how energy transforms and does work is key to innovations in technology, engineering, and science.
Calculations
There are several key calculations related to energy and work that are important to understand:
Calculating Kinetic and Potential Energy
Kinetic energy is energy associated with motion and is calculated using the formula:
Kinetic Energy = 1/2 x mass x velocity^2
Potential energy is stored energy and depends on the position or configuration of an object. There are several types of potential energy including gravitational potential energy which can be calculated using:
Gravitational Potential Energy = mass x gravitational acceleration (g) x height
Calculating Work Done
Work is done when a force causes motion. The amount of work done by a force can be calculated using:
Work = Force x Distance
The units for work are Joules (J) in the metric system and Foot-pounds (ft-lb) in the English system. The amount of work done is directly proportional to the distance over which the force acts.
Conclusion
In summary, energy and work are fundamentally different concepts in physics, despite being closely related.
Energy is the capacity to do work and can exist in various forms like kinetic, potential, thermal, chemical, etc. It is measured in joules and cannot be created or destroyed, only transformed from one form to another. Work, on the other hand, is the process of transferring energy from one object or system to another through the application of a force over a distance. Work is measured in joules as well but represents energy expenditure, not storage.
While work can convert energy from one form to another, it does not create or destroy energy. The total energy before and after work is performed remains constant, as stated by the law of conservation of energy. Knowing how to calculate and distinguish between energy and work is essential for analyzing mechanical, electrical, and thermal systems.