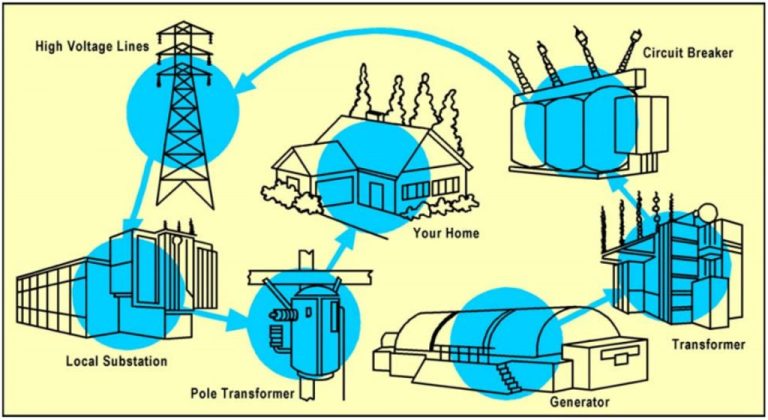
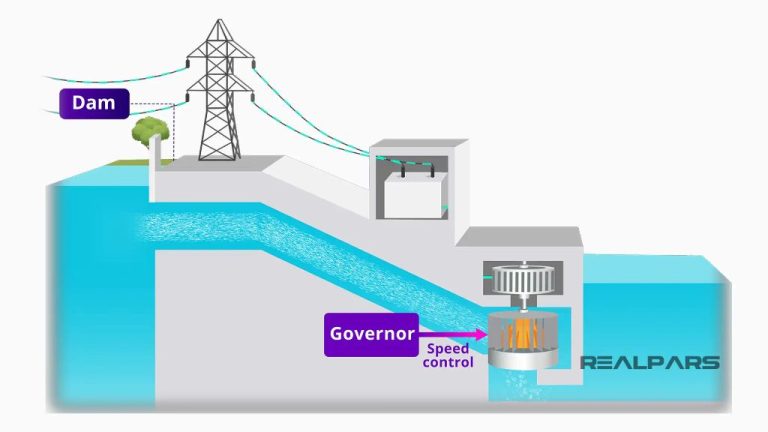
Does It Take More Energy To Make Hydrogen Than It Produces?
Introduction
The hydrogen economy has gained significant interest in recent years as a potential clean energy source to help address climate change. Hydrogen is considered a versatile energy carrier that can be used for transportation, electricity generation, and energy storage. However, one of the major challenges facing the hydrogen economy is the efficiency of hydrogen production.
Most hydrogen today is produced from fossil fuels through processes like steam methane reforming which have high energy inputs. In fact, some analyses show it can take more energy to produce hydrogen than the energy ultimately obtained from using it (Kar, 2023). This makes the overall efficiency and sustainability of hydrogen questionable.
This article provides an overview of the inputs and outputs in hydrogen production to analyze the efficiency and determine if it does indeed take more energy to make hydrogen than the energy it can provide. The outlook for improving efficiency through renewable production methods is also examined.
How Hydrogen is Made
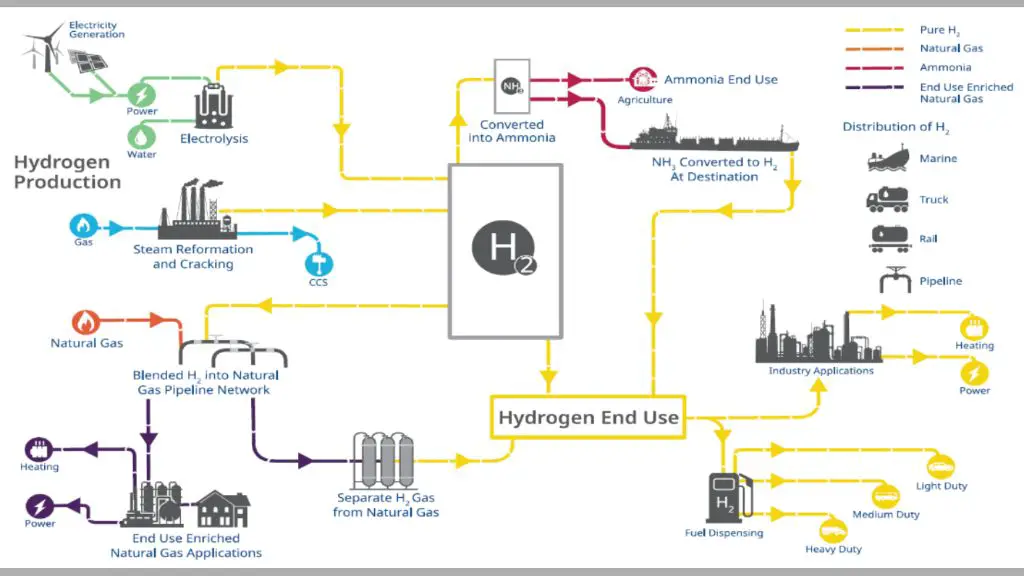
Hydrogen is mainly produced through two methods – steam reformation of natural gas, and water electrolysis. Steam reformation involves reacting high temperature steam with a hydrocarbon fuel like natural gas to produce hydrogen gas. This accounts for around 95% of global hydrogen production currently [1]. The other 5% comes from electrolysis, which uses electricity to split water molecules into hydrogen and oxygen. While fossil fuel-based hydrogen production emits greenhouse gases, electrolysis using renewable electricity offers a clean way to produce hydrogen.
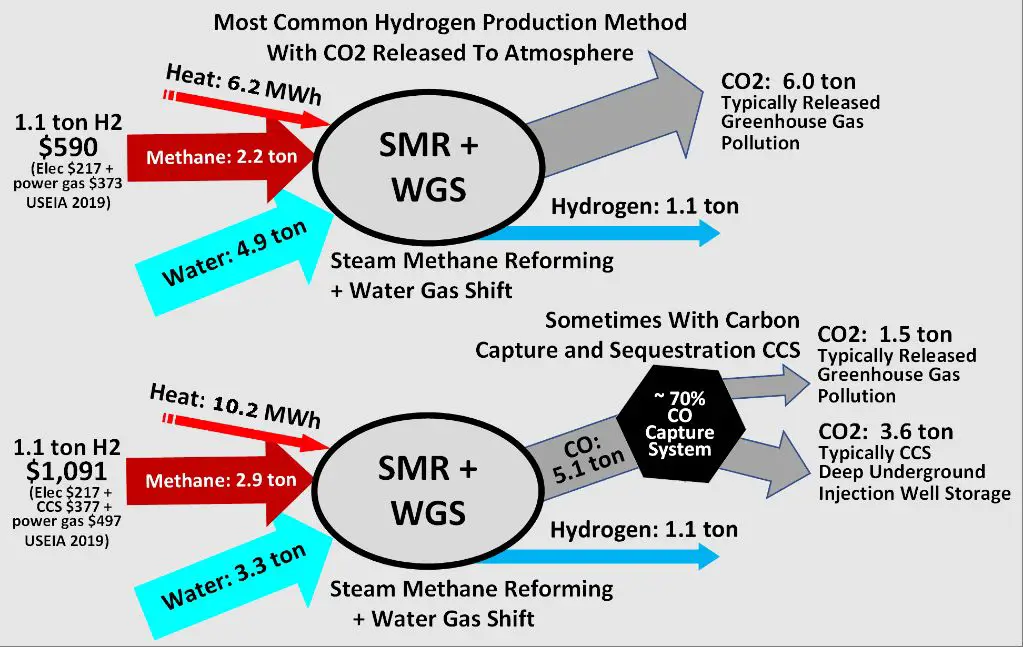
Some other less common methods of hydrogen production include partial oxidation of oil, coal gasification, biomass gasification, and photoelectrochemical water splitting. But steam reformation and electrolysis are by far the predominant methods used today [2].
Energy Inputs for Hydrogen Production
The primary energy inputs for hydrogen production include electricity, heat, and chemical feedstocks. The amount of energy required depends largely on the production method.
Electrolysis, which uses electricity to split water into hydrogen and oxygen, requires a significant energy input. According to a 2011 study, “the energy required to produce 1 kg of hydrogen [via electrolysis] is equivalent to the electricity generated by the combustion of approximately 5.6 kg of hydrogen.” (Source)
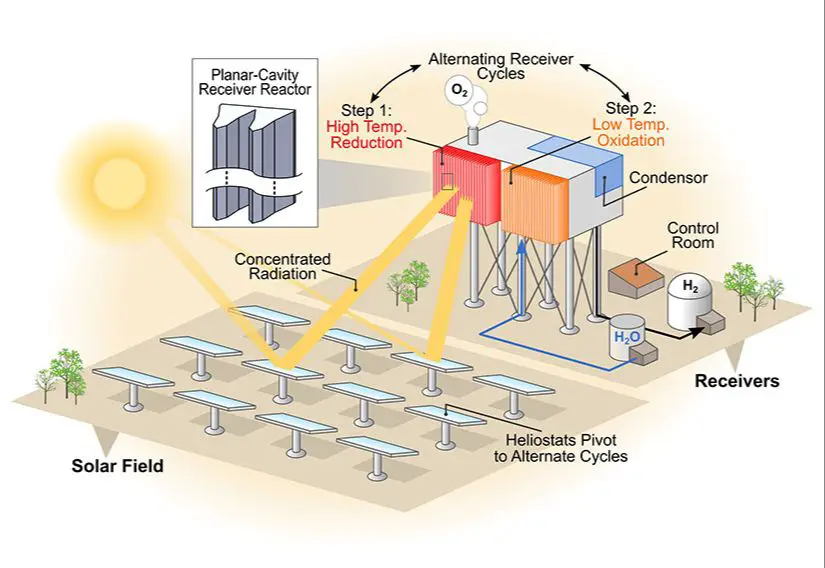
Thermochemical production using heat and chemical reactions requires high temperatures, often over 850°C. This necessitates a substantial heat input. According to one source, thermochemical cycles require 2-3 times more energy than low-temperature electrolysis. (Source)
Reforming of natural gas uses heat, steam, and methane as inputs. This generates carbon emissions, but requires less direct energy input than electrolysis or thermochemical approaches.
Overall, electrolysis and thermochemical production require significant electricity and heat to split water and free the hydrogen. Natural gas reforming needs less energy input, but creates carbon emissions.
Potential Energy Output from Hydrogen
When hydrogen is used as an energy carrier, for instance in a fuel cell, it has the potential to release substantial amounts of energy. This occurs through a chemical reaction between hydrogen and oxygen inside the fuel cell which produces electricity with water and heat as byproducts.
The energy output from hydrogen depends on the specific application. In hydrogen fuel cells for vehicles, the theoretical maximum conversion efficiency is around 80% based on the fuel’s higher heating value. However, practical conversion efficiencies tend to be 40-60%. This is still considerably higher than the 20-35% efficiency seen in conventional internal combustion engines (https://slideplayer.com/slide/7685492/).
For larger stationary fuel cells, efficiency can potentially reach up to 85%. But again, real-world systems operate at 50-60% efficiency. Nevertheless, this is much better than the average efficiency of fossil fuel power plants, which is around 40% (https://www.slideserve.com/quincy/introduction-to-hydrogen-fuel-cells).
So in summary, hydrogen has the potential to release substantial amounts of energy, at efficiencies notably higher than traditional combustion technologies. When produced renewably, hydrogen provides a clean and efficient energy carrier.
Efficiency of Hydrogen Production
The efficiency of hydrogen production refers to the ratio of the energy output of the produced hydrogen to the energy inputs required in its production process. Calculating the net energy gain or loss in hydrogen production involves comparing the higher heating value of hydrogen to the cumulative energy required for feedstock, electricity, heat, and other inputs across the full production pathway (Burton, 2021).
For water electrolysis powered by renewable electricity, studies show a wide efficiency range of 50-80%, with advanced technologies reaching optimized rates above 70% (Interesting Engineering, 2023). On the other hand, steam methane reforming of natural gas has a typical efficiency around 75-80%, but with higher carbon emissions. Overall, calculations show that renewably-produced hydrogen via water electrolysis can achieve a small but positive net energy gain (Burton, 2021).
Improving the production efficiency is a major focus in hydrogen research. This includes optimizing electrolyzer design, finding more efficient catalysts, integrating heat recovery, and utilizing renewable electricity inputs (ScienceDirect, 2022). With further efficiency gains, green hydrogen production is expected to achieve greater net energy yields and become more cost-competitive.
Improving Efficiency
Hydrogen production can be made more efficient in several ways:
Research shows that improving the design and components of steam methane reforming can increase efficiency. This includes optimizing heat integration and recovery, using better catalysts, and integrating membrane separation. Efficiency gains of over 10% have been demonstrated.
For electrolysis, advancements in electrode and electrolyte materials, cell architecture, and operating conditions allow electrolyzers to convert more of the input electricity into hydrogen. Efficiencies over 80% are achievable.
Efficiency can also be improved by using waste heat or renewable electricity sources like solar and wind. This provides the energy input at little to no direct cost.
Overall, continued research and development across production methods focuses on increasing efficiency to lower costs and make hydrogen more competitive.
Renewable Hydrogen Production
Hydrogen can be produced from renewable energy sources like solar, wind, hydropower, geothermal, and biomass. Using renewable energy addresses the efficiency and emissions issues associated with fossil fuel-based hydrogen production. There are several methods for using renewables to produce hydrogen:
Renewable Hydrogen Production provides a comprehensive analysis of renewable energy-based hydrogen production. Through simulation analysis and experimental testing, they evaluated options like using excess renewable electricity for electrolysis, biomass gasification, and biological hydrogen production with algae and bacteria.
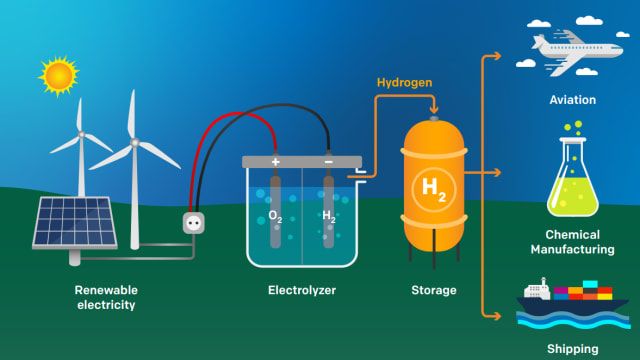
The most common method is electrolysis powered by renewable electricity. Excess solar or wind energy that would otherwise be curtailed can be used to split water into hydrogen and oxygen. With 100% renewable power, this results in zero-emissions hydrogen.
Biohydrogen utilizes organic matter like crop residues or waste to produce hydrogen through thermochemical or biological processes. This provides a way to recycle waste into clean fuel.
Overall, hydrogen from renewables has the potential to provide clean energy storage and mitigatecurtailment issues for intermittent renewable generation. With continued technology development and cost reductions, renewable hydrogen could play a major role in decarbonization.
Future Outlook
The future looks bright for renewable hydrogen production. Companies like Air Liquide are investing heavily in large-scale renewable hydrogen projects powered by electrolysis using wind and solar energy (Air Liquide, 2023). Electrolysis splits water into hydrogen and oxygen using electricity, and when paired with renewable energy sources, can produce hydrogen without any carbon emissions.
Industry experts predict rapid growth in renewable hydrogen in the coming decades as costs continue to fall and more projects come online (SAAB RDS, 2023). The European Union in particular sees great potential for renewable hydrogen to help achieve its climate goals and is developing supportive policies and incentives (Nunez-Jimenez, 2022). With continued technology improvements and economies of scale, renewable hydrogen is expected to reach cost parity with conventional production within 10-15 years.
The prospects for efficient, low-carbon renewable hydrogen look increasingly positive. With the right investments and policy support, renewable hydrogen can emerge as a major sustainable energy source and enable the decarbonization of hard-to-abate sectors of the economy.
Conclusions
In summary, the findings on the energy balance of hydrogen production show that there are efficiency losses, but hydrogen can provide a viable energy source when renewable energy is used for production. Early analysis by Bossel in 2006 suggested hydrogen FCVs may have poor efficiency compared to other options [1]. However, more recent research in 2021 highlights that with renewable electricity, hydrogen production can achieve efficiency rates exceeding 70% [2]. While fossil fuel-based hydrogen production is less efficient, transitioning to electrolysis with solar, wind, or hydro power can make hydrogen a relatively efficient carrier of renewable energy. With improvements in production and fuel cell technology, hydrogen’s efficiency profile is likely to increase further. Thus, hydrogen can potentially serve as an important energy storage and transportation mechanism in a renewable energy system, despite some efficiency losses in production.
References
Hydrogen Production: Processes, Technologies and Development Trends. Zini, G. and Tartarini, P. MDPI. Retrieved from https://www.mdpi.com/1996-1073/10/11/1521
Life Cycle Assessment of Hydrogen Production via Natural Gas Steam Reforming. Spath, P and Mann, M. National Renewable Energy Laboratory. Retrieved from https://www.nrel.gov/docs/fy99osti/25106.pdf
Hydrogen Production: Fundamentals and Case Study Summaries. National Renewable Energy Laboratory. Retrieved from https://www.nrel.gov/docs/fy03osti/35070.pdf