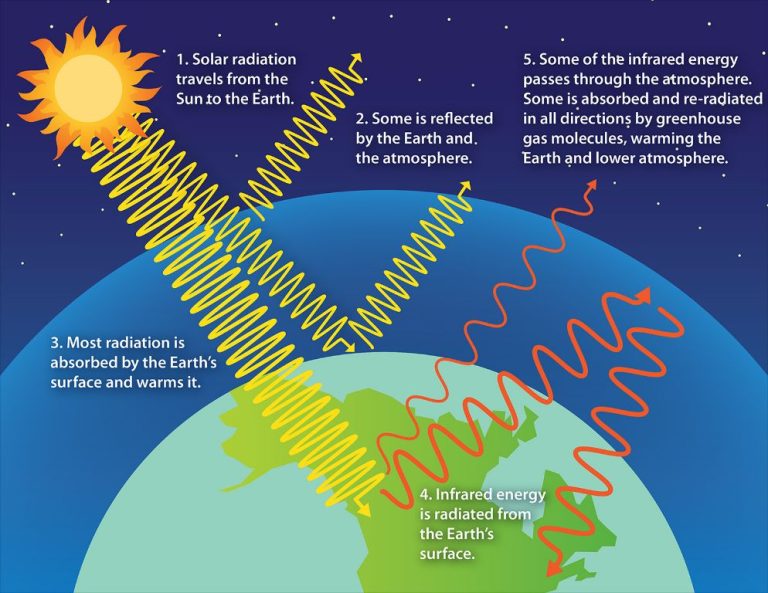
Does Energy Depend On Temperature?
Energy and temperature are fundamental concepts in physics that are closely linked. While they represent different physical properties, understanding the relationship between energy and temperature is key to explaining many important natural phenomena and technological processes.
Energy is defined as the capacity to do work or transfer heat. It exists in many forms such as kinetic, potential, thermal, chemical, nuclear and more. Temperature is a measure of the average kinetic energy of microscopic particles that make up a substance. As temperature increases, particle motion increases, which results in an increase in the total kinetic energy and therefore the total energy of the system.
The connection between energy and temperature reveals itself in many areas of physics and engineering, from heat engines to quantum physics. This article will provide an in-depth look at how energy and temperature interact across various domains.
Definition of Energy
Energy is the ability to do work or produce heat. There are two main forms of energy: potential energy and kinetic energy.
Potential energy is stored energy based on an object’s position or arrangement. For example, a ball held at a height above the ground has gravitational potential energy due to the earth’s gravity pulling it downwards. This energy can be released if the ball falls or is thrown. Other examples of potential energy include chemical energy stored in molecules and elastic energy stored in compressed or stretched springs.
Kinetic energy is energy associated with motion. Any object that is moving has kinetic energy. The amount of kinetic energy depends on the object’s mass and velocity. For example, a car driving down the road and a baseball flying through the air both have kinetic energy. Other examples include the motion of electrons, sound waves, and electromagnetic radiation.
Energy can be transferred between potential and kinetic forms. An object at a height has potential energy that can be converted into kinetic energy as it falls. The kinetic energy of motion can be converted into potential energy if an object is lifted upwards. The law of conservation of energy states that the total energy in a closed system remains constant.
Definition of Temperature
Temperature is the measure of the average kinetic energy of molecular motion in a substance. On an atomic scale, temperature represents the motion of particles. As particles move faster they have greater kinetic energy, which corresponds to higher temperatures. In this sense, temperature is a basic indicator of the thermal energy at the molecular level.
Thermal Energy
Thermal energy refers to the internal energy within a system that is associated with the random motion of molecules. The higher the temperature of a substance, the greater the molecular movement and kinetic energy of those molecules. When a substance is heated, its molecules vibrate and move more rapidly, increasing the thermal energy. This increased molecular motion corresponds to an increase in temperature.
On a microscopic scale, increasing temperature causes molecules to vibrate faster and move more, hitting into each other and transferring kinetic energy. The greater kinetic energy of the molecules is observed macroscopically as increased thermal energy. This relationship between temperature and thermal energy underlies many thermodynamic processes and properties of matter.
Thermal Energy Increases with Temperature
When an object’s temperature increases, the thermal energy of the object also increases. This is because temperature is a measure of the average kinetic energy of the molecules in a substance. As an object gets hotter, its molecules vibrate and move faster, colliding more frequently with each other and their container. These molecular collisions transfer kinetic energy throughout the substance, increasing the total thermal energy.
For example, as water is heated from cold to lukewarm to boiling, the water molecules move faster and faster. This molecular motion is perceived as thermal energy. The greater the molecular motion, the higher the temperature and thermal energy of the water. This relationship between temperature and thermal energy applies to all matter, whether solid, liquid or gas.
Thermal energy is often referred to as heat. While heat and temperature are related, they are distinct properties. Temperature measures the average kinetic energy of molecules, whereas heat refers to the total thermal energy transferred between substances due to temperature difference. As an object’s temperature rises, its increasing molecular motion enables greater heat transfer and thermal energy capacity.
Phase Changes and Latent Heat
When matter undergoes a phase change, such as from a solid to a liquid or from a liquid to a gas, energy is absorbed or released while the temperature remains constant. This energy is known as latent heat. For example, when ice melts to become liquid water at 0°C, it absorbs latent heat. The latent heat required to melt ice is known as the heat of fusion. Similarly, when water boils and becomes steam at 100°C, it absorbs the latent heat of vaporization.
Conversely, when steam condenses into liquid water, or when water freezes into ice, the same amount of latent heat is released. Therefore, phase changes require an input or output of energy even though the temperature does not change during the process. The amount of latent heat absorbed or released depends on the mass, type of substance, and the phase change it is undergoing. This behavior highlights the fact that temperature alone does not dictate the energy of a system – the phase and bonding between molecules is also critically important.
Heat Transfer
Heat transfer is the exchange of thermal energy between substances or systems due to a temperature difference. There are three main types of heat transfer:
Conduction – The transfer of heat between substances in direct physical contact. It occurs between atoms and molecules and can only take place in solids, liquids and gases. Conduction depends on the temperature gradient and the properties of the materials in contact.
Convection – The transfer of heat by the movement of heated fluid from one place to another. Fluid flows help transfer heat from hotter regions to cooler ones. Natural convection occurs due to density differences while forced convection is due to external factors like fans or pumps.
Radiation – The transfer of heat via electromagnetic waves or photons. It can travel through empty space unlike conduction and convection. All objects emit thermal radiation depending on their temperature. Hotter objects emit more radiation than cooler ones.
Temperature and Kinetic Energy
The molecules that make up matter are constantly in motion. As the temperature of matter increases, the molecules move faster as they gain kinetic energy. Kinetic energy is the energy of motion. At higher temperatures, molecules vibrate rapidly and move quickly as they collide with each other. This greater molecular motion at higher temperatures corresponds to an increase in kinetic energy.
For example, as water is heated from a solid ice state to a liquid state and eventually to a gaseous state, the water molecules gain kinetic energy and move faster. This increase in molecular motion translates to a direct increase in temperature. Therefore, temperature serves as a measure of the average kinetic energy of molecules within matter.
Exceptions
While temperature and energy are closely related in most scenarios, there are some notable exceptions worth mentioning:
Negative temperatures: Typically temperature is measured on scales like Celsius and Fahrenheit, where 0 is the lowest theoretical limit. However, in some quantum systems it’s possible to achieve “negative” temperatures below absolute zero. In these exotic systems, an increase in energy can correspond to a decrease in temperature.
Zero-point energy: Even at a temperature of absolute zero, quantum physics indicates that particles retain a residual amount of energy called zero-point energy. This baseline of energy exists regardless of temperature, demonstrating that energy does not always depend directly on temperature.
So while in most everyday situations, energy and temperature have a strong correlation, exotic quantum effects can lead to unusual exceptions where energy changes do not map directly onto temperature changes.
Conclusion
In summary, we have seen that there is a close relationship between temperature and energy. As temperature increases, the thermal energy and kinetic energy of atoms and molecules also increases. This is because temperature is a measure of the average kinetic energy of particles in a substance. The higher the temperature, the faster the particles are moving on average.
This relationship explains many everyday phenomena. For example, heating water increases its temperature, which gives the water molecules more kinetic energy. This allows them to break free from each other as a gas during boiling. Temperature also impacts phase changes between solid, liquid, and gas states. As more energy is added, particles gain enough kinetic energy to overcome intermolecular forces and change state.
While the temperature-energy relationship does not always hold true in every scenario, it is a fundamental concept in thermodynamics. Understanding how temperature and energy are connected provides great insight into the behavior of matter and energy transfers in our universe.