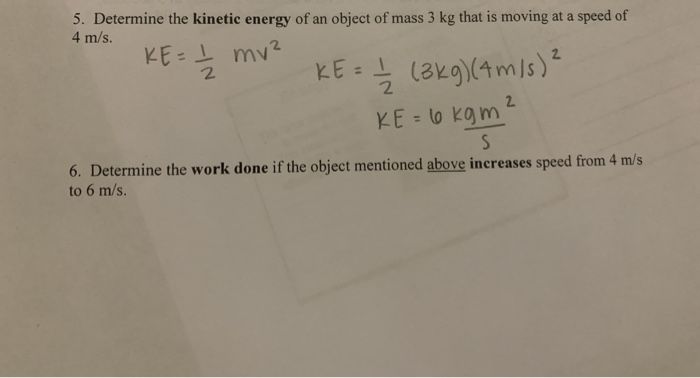Can Energy Can Be Transferred?
Energy transfer refers to the process by which energy is passed from one object, substance, or system to another. According to the law of conservation of energy, the total energy in a closed system remains constant. While energy cannot be created or destroyed, it can be transformed from one form to another. There are many different forms that energy can take, including kinetic, potential, thermal, electrical, chemical, nuclear and more. Energy transfers happen continuously as energy flows between objects and systems.
The laws of thermodynamics describe how energy transfers work. The first law states that energy is always conserved – it cannot be created or destroyed, only transformed from one form to another. The second law states that in any energy transfer, some amount of energy is lost to heat, resulting in a less ordered, lower-energy state. While energy remains constant, its ability to do work decreases with each transfer due to these thermal losses.
This overview will examine the different forms energy can take and how energy transfers between them according to the laws of physics.
Kinetic Energy
Kinetic energy is the energy of motion. An object that has motion, whether it is vertical or horizontal motion, has kinetic energy. Some examples of kinetic energy include:
- A bouncing ball has kinetic energy when it bounces up and down.
- A person walking or running has kinetic energy.
- A bicycle in motion has kinetic energy.
Kinetic energy can be transferred when objects collide with each other. For example, when a moving ball hits a stationary ball, the first ball transfers some of its kinetic energy to the second ball, causing it to move. The friction between moving surfaces can also cause kinetic energy to be transferred as heat energy. Overall, kinetic energy is directly related to the mass and velocity of an object – the more massive and faster an object moves, the greater its kinetic energy.
Potential Energy
Potential energy is the stored energy in an object due to its position or chemical composition. For example, a book sitting on a table has potential energy due to gravity. If it falls off the table, the potential energy is converted into kinetic energy as the book speeds up while falling. Some examples of potential energy include:
- Gravitational potential energy – energy stored in an object due to height, such as a rollercoaster at the top of a hill or water held behind a dam.
- Elastic potential energy – energy stored in stretched or compressed objects like springs or rubber bands.
- Chemical potential energy – energy stored in the chemical bonds of substances like gasoline, batteries, and food.
Gravitational potential energy can be transferred when an object falls or slides down a slope. The height determines the amount of potential energy. As the object drops, this potential energy is transferred into kinetic energy. Chemical potential energy can be transferred in chemical reactions. The chemical bonds in the reactants are broken, releasing energy that becomes kinetic energy of the products or thermal energy.
Heat Energy
Heat energy is the transfer of thermal energy between objects or systems due to a temperature difference. All matter contains internal energy, which is the kinetic and potential energy of all the atoms and molecules within it. This internal energy manifests itself as thermal energy, which is the total kinetic and potential energy of the particles that make up a substance.
Heat energy transfers from high temperature objects to lower temperature objects when they come into contact, seeking thermal equilibrium. This transfer occurs in three main ways: conduction, convection, and radiation. Conduction is the transfer of heat between objects in direct contact. Convection is heat transfer via the motion of fluids. Radiation is the emission of electromagnetic waves from an object due to its internal thermal motion; these waves travel through space and raise the thermal energy of anything they interact with.
Common examples of heat transfer include heating a pot of water on the stove, which adds thermal energy via conduction from the burner to the pot. The motion of the heated water rising and cooler water sinking is convection. A fire radiates heat that we feel as the electromagnetic waves strike our skin. Heat energy is an important form of energy in everyday phenomena and energy systems.
Electrical Energy
Electrical energy refers to energy derived from the movement of electrons. It involves the flow of electrons through a conductor like a metal wire or cable. The electrons transmit energy that powers machines and devices. Some key things about electrical energy:
– It is a secondary form of energy produced from the conversion of other sources like coal, natural gas, solar, wind, hydroelectric, or nuclear energy.
– Electrical generators and turbines convert mechanical energy into electrical energy.
– Voltage is the force or pressure driving the electric current. Current is the amount of electric charge flowing past a point over a certain time.
– Circuits provide a closed conducting loop for electrons to flow through. Amperes measure the electric current or number of electrons passing through a point in the circuit per second.
– Electrical energy can be converted into other forms like heat, light, or motion to power appliances and devices.
– It is transported efficiently over long distances via power grids and distribution systems.
– Electrical energy follows the law of conservation of energy. The amount of electrical energy converted from a source equals the amount that powers devices, minus any energy lost as heat in the system.
Chemical Energy
Chemical energy is the potential energy stored in the chemical bonds between atoms and molecules. It is the energy released when a chemical reaction occurs. For example, the food we eat contains chemical energy that is released through metabolic reactions to power our cells. Batteries also store chemical energy in the bonds of their chemicals, which is converted to electrical energy to power devices.
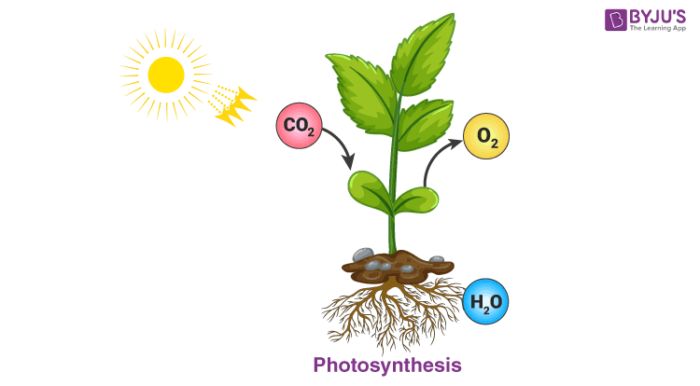
Chemical energy can be transferred when the bonds in molecules are broken or formed. Breaking bonds requires energy input, while forming bonds releases energy. For example, plants use the radiant energy of sunlight along with carbon dioxide and water to photosynthesize glucose and oxygen. This process stores chemical energy in the glucose molecules’ bonds. When we eat and digest this glucose, chemical reactions break the bonds to release energy that our bodies use. The combustion of fuels like wood, oil, or natural gas also releases large amounts of chemical energy through oxidation reactions.
Radiant Energy
Radiant energy is energy that is transmitted by electromagnetic waves, including visible light, radio waves, gamma rays, and X-rays. It is a form of kinetic energy since electromagnetic waves carry energy as they oscillate and travel through space. Some examples of radiant energy include:
- Sunlight
- Heat and light from a fire
- Energy from radio waves, microwaves, infrared radiation
- X-rays and gamma rays
Radiant energy can transfer between objects that are not in direct contact with each other. The electromagnetic waves carry energy that can be absorbed by matter. For example, the sun’s radiant energy travels to Earth through the vacuum of space via electromagnetic waves. When these waves reach Earth, they pass through the atmosphere and are absorbed by land, water, plants, etc, heating the planet. Radiant energy transfers thermal energy without requiring direct contact between the source (the sun) and the recipient (Earth’s surface). This allows heat to transfer through open space, rather than just through matter.
Sound Energy
Sound energy is the energy carried by sound waves. When an object vibrates, it creates vibrations in the air particles around it, forming a sound wave that carries energy through the air. The energy in sound is transferred through the vibration of air molecules rather than the actual movement of air.
Some examples of sound energy include:
- Music from instruments like guitars, pianos, or drums
- Noises like clapping, whistling, shouts, etc.
- Sonic booms from supersonic aircraft
- Bat or dolphin echolocation
The vibrations of sound waves can make objects vibrate as well. This allows sound energy to be transferred between the source and the vibrating objects. For example, when you play music on loud speakers, the speakers vibrate and transfer energy into the air. The sound waves then carry this energy until they reach your ears, vibrating your eardrums and allowing you to hear the music.
Nuclear Energy
Nuclear energy is the energy stored in the nucleus of an atom. It is released when the nucleus is split apart (through fission) or fused together (through fusion). Nuclear energy can be transferred when unstable nuclei decay radioactively, resulting in the emission of ionizing radiation.
Some examples of nuclear energy include:
- The energy produced in nuclear power plants comes from nuclear fission reactions splitting uranium or plutonium atoms.
- The sun produces energy through nuclear fusion reactions fusing hydrogen atoms together.
- Radioactive decay from unstable isotopes like uranium-238 and thorium-232 produce radiant energy.
Nuclear energy is extremely efficient – nuclear fission releases nearly a million times more energy per atom than chemical reactions like burning fossil fuels. However, nuclear power also carries risks, like the potential for severe accidents and long-lived radioactive waste.
Conclusion
In conclusion, there are many different forms that energy can transfer between. Some of the most common forms of energy transfer include kinetic energy, potential energy, heat energy, electrical energy, chemical energy, radiant energy, sound energy, and nuclear energy.
The ability for energy to transfer between different forms is extremely important in our everyday lives and world. For example, chemical energy in food and gasoline is able to transfer into kinetic energy to move our bodies and vehicles. Electrical energy can power our homes and devices by first being converted from sources like coal, natural gas, solar, wind, and nuclear power.
Energy transfers allow us to harness natural forms of energy like wind, water, and sunlight in order to generate electricity. The study of energy transfers has enabled many key inventions and technologies that support modern life. Overall, the fact that energy can change forms allows it to be harnessed in ways that provide essential power for human society.