Can Energy Be Converted From One To Another?
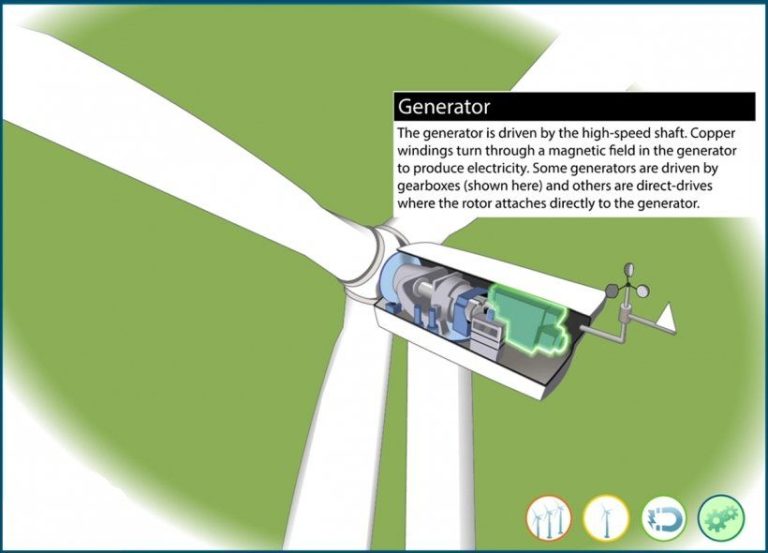
Energy conversion is the process of changing energy from one form to another. For example, a wind turbine converts the kinetic energy of wind into electrical energy. The law of conservation of energy states that energy can neither be created nor destroyed, it can only be converted from one form to another. This means that the total energy in a closed system always remains constant. While energy conversions can result in the loss of usable energy, the total energy is conserved according to this fundamental law of physics.
Forms of Energy
There are six main forms that energy can take:
Potential Energy
Potential energy is stored energy due to position or composition. For example, a ball held up in the air has gravitational potential energy that can turn into kinetic energy when released. Chemical potential energy is energy stored in the bonds between atoms and molecules that is released in chemical reactions.
Kinetic Energy
Kinetic energy is energy of motion. A moving object, like a ball rolling down a hill, has kinetic energy. The faster it moves, the more kinetic energy it has.
Thermal Energy
Thermal energy comes from the motion of atoms and molecules. The more motion, the higher the thermal energy which we perceive as heat. Thermal energy can transfer between objects of different temperatures.
Electrical Energy
Electricity comes from the movement of electrons. Materials called conductors, like copper wire, allow electrons to freely flow creating electric current. Electrical energy can be generated and stored for later use.
Chemical Energy
Chemical energy is stored in the bonds between atoms and molecules and released in chemical reactions. Foods, gasoline, batteries and living organisms store chemical energy that is converted to other forms of energy.
Nuclear Energy
Nuclear energy comes from processes within an atom’s nucleus like radioactive decay and nuclear fusion/fission. Nuclear reactions release huge amounts of energy from small amounts of matter.
Energy Conversions in Nature
Energy is continuously converted from one form to another in the natural world through various biological processes. Three key examples of energy conversions in nature are photosynthesis, cellular respiration, and food chains.
Photosynthesis is the process by which plants convert light energy from the sun into chemical energy in the form of glucose. This conversion takes place in plant cells, which use chlorophyll to absorb sunlight and convert it into energy through a series of chemical reactions. The end result is carbohydrates that the plant uses for energy and growth.
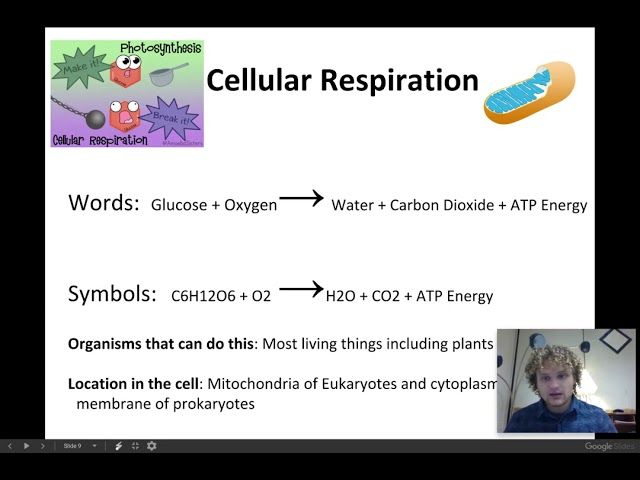
Cellular respiration is the opposite process, in which cells convert the chemical energy stored in food into a form they can use. Through a series of steps including glycolysis and the Krebs cycle, glucose and other nutrients are broken down and their energy is transferred to molecules of ATP. The energy stored in ATP is then used to power cellular processes.
In food chains, energy is passed from one organism to another. Producers such as plants convert sunlight into chemical energy through photosynthesis. Consumers then obtain this stored energy by eating plants and other animals. At each step throughout the food chain, some energy is lost as heat, requiring a continuous input of sunlight to fuel the ecosystem.
These examples demonstrate how energy conversions enable natural processes like plant growth, animal metabolism, and nutrient cycling. The continuous cycling of energy between sunlight, chemical bonds, and food webs powers life on Earth.
Man-Made Energy Conversions
Humans have harnessed various forms of energy to perform useful work through ingenious inventions and machines. Power plants, engines, and batteries are prime examples of human-made systems that convert energy from one form to another.
Power plants convert the chemical energy stored in fuel sources like coal, natural gas, or uranium into electricity. The electricity can then be used to power homes, businesses, and cities. Coal and natural gas plants boil water into steam, which spins a turbine connected to a generator to produce electricity. Nuclear plants use nuclear fission to create heat and spin turbines.
Gasoline and diesel engines in vehicles, machinery, and generators also convert the chemical energy in fuel into mechanical energy and motion. Smaller engines use the combustion of fuel to move pistons and drive a crankshaft. This rotational kinetic energy is then used to propel wheels or drive other mechanisms.
Batteries are electrochemical devices that convert between chemical and electrical energy. Primary batteries irreversibly transform chemical energy into electricity through spontaneous reactions. Rechargeable batteries can reverse the reaction to reconvert electrical energy into stored chemical energy. Common battery types include lead-acid, lithium-ion, and nickel-cadmium.
These and many other ingenious inventions allow humans to harness energy from nature and convert it into useful forms of power for work, transportation, electricity, and more. Energy conversions enable modern civilization, technology, and improving standards of living.
Efficiency of Conversion
When energy is converted from one form to another, some amount of useful energy is always lost in the process. This principle is known as the second law of thermodynamics, which states that the entropy (disorder) of the universe always increases over time. In simple terms, this means that no energy conversion is 100% efficient.
For example, when coal is burned to generate electricity in a thermal power plant, a large amount of heat energy is produced as a byproduct and released into the environment. This waste heat energy cannot be fully captured or converted into useful electrical energy. Similarly, when gasoline is used to power a car engine, roughly 70% of the energy is lost as heat and exhaust. Only about 25% of the gasoline energy actually goes into powering the wheels.
The maximum possible efficiency of a energy conversion process depends on many factors like the temperature differential, the properties of the materials involved, engineering limitations, etc. But there will always be some percentage of energy wasted. This inevitable loss of energy during conversion is a fundamental limit rooted in the laws of physics and thermodynamics.
Understanding these theoretical limits helps engineers design better systems to maximize efficiency. But no real-world energy conversion can be perfect. There will always be some waste, in line with the second law of thermodynamics which governs our universe.
Famous Equations
Two of the most famous equations in physics describe the relationship between different forms of energy and matter:
E = mc^2
This equation, developed by Albert Einstein in 1905, shows that energy (E) is equal to mass (m) times the speed of light (c) squared. It demonstrates that mass and energy are equivalent and can be converted into one another.
PV = nRT
The ideal gas law, also known as the equation of state for a perfect gas, shows the relationship between pressure (P), volume (V), amount of substance (n), universal gas constant (R), and temperature (T) for a gas. This equation demonstrates how the internal energy of a gas relates to its pressure, volume, and temperature.
These two famous equations illustrate that energy can be readily converted from one form into another, such as between mass and energy or between the internal energy of a gas and its pressure/volume/temperature.
Real-World Applications
Energy conversions occur constantly in our everyday lives. Here are some common examples:
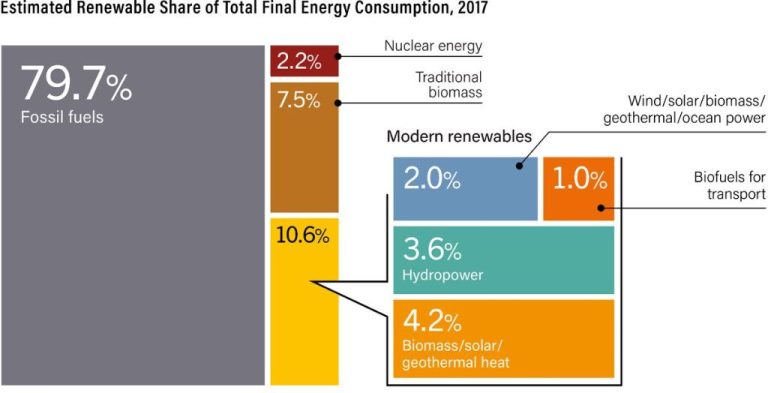
Electricity generation – Most electricity is generated by converting the mechanical energy of turbines at power plants into electrical energy that is then distributed to homes and businesses. The turbines are typically powered by the chemical energy in fossil fuels, nuclear energy, or the kinetic energy of wind or water.
Transportation – Gasoline and other fuels contain stored chemical energy that is converted into kinetic energy that propels cars, trucks, planes, and ships. Hybrid and electric vehicles also use electrical energy stored in batteries to power electric motors.
Home appliances – Devices like refrigerators, microwaves, and hairdryers all convert electrical energy into other useful forms – thermal energy for heating and cooling, radiant energy for cooking, mechanical energy for mixing and blowing air, etc.
Solar panels – Solar photovoltaic panels convert radiant light energy from the sun directly into electrical energy.
Batteries – Chemical reactions within batteries convert stored chemical energy into electrical energy.
Digestion – The body converts the chemical energy stored in food into thermal and mechanical energy to maintain body temperature and power movement.
Incandescent light bulbs – Electrical energy is converted into radiant light energy and thermal energy (heat). LED and fluorescent bulbs are more efficient as more of the energy is converted into light.
Pros and Cons
Energy conversion has many advantages, but also some disadvantages that should be considered.
Pros
Being able to convert energy from one form to another provides us with more options and flexibility. For example, converting chemical energy in fossil fuels to mechanical energy allows us to power vehicles for transportation. Converting solar energy into electrical energy through photovoltaic panels gives us a renewable energy source.
Energy conversion allows us to transform energy into the most useful and applicable form for the task at hand. We can store energy in compact chemical or potential forms, then convert it to electricity when we need power. This makes energy more transportable, controllable, and accessible.
Some forms of energy like electricity are higher quality and more valued than others like heat. Being able to convert lower quality waste heat to electricity makes the energy more usable and valuable.
Cons
The process of energy conversion often generates waste heat and inefficiencies. Much of the initial energy is lost or dissipated. For example, internal combustion engines have an efficiency of only about 30%. This wasted energy can contribute to problems like excessive heat and environmental issues.
Energy conversions require technology and infrastructure which can be complex and expensive, like electrical generator plants or solar arrays. The facilities, materials, and maintenance associated with conversion processes have high costs.
Some conversion processes produce harmful byproducts like greenhouse gas emissions and nuclear waste. These can contribute to environmental pollution and ecological damage. Careful consideration and management of byproducts is needed.
Latest Research
Scientists around the world continue to push the boundaries of our understanding of energy conversion. Here are some notable recent discoveries and breakthroughs:
Researchers at MIT developed a new thermoelectric material that is over three times more efficient at converting heat to electricity. This could dramatically improve the efficiency of power generation from waste heat.
German scientists found a way to convert over 40% of light energy to hydrogen fuel using photocatalysts. This new technique could unlock cleaner and more sustainable fuel production.
A team at Caltech recently demonstrated a new solar cell design that can convert electricity to light and back at an unprecedented efficiency of over 50%. Such reversible conversion could enable new technologies.
Stanford engineers created a novel nanoparticle-based material that can convert lower-grade heat to electricity with twice the efficiency of conventional methods. This could allow more reuse of waste heat.
Researchers in Singapore are developing innovative biological methods to convert carbon dioxide and methane to liquid fuels using bacteria. This bioconversion aims to create a carbon-neutral fuel alternative.
Conclusion
Through our discussion, we have seen that energy is a fundamental property of the universe that can take on different forms such as kinetic, potential, thermal, chemical, nuclear and more. Energy allows work to be accomplished and heat to be transferred between objects. While energy can freely convert between these different forms, it can never be created or destroyed according to the law of conservation of energy.
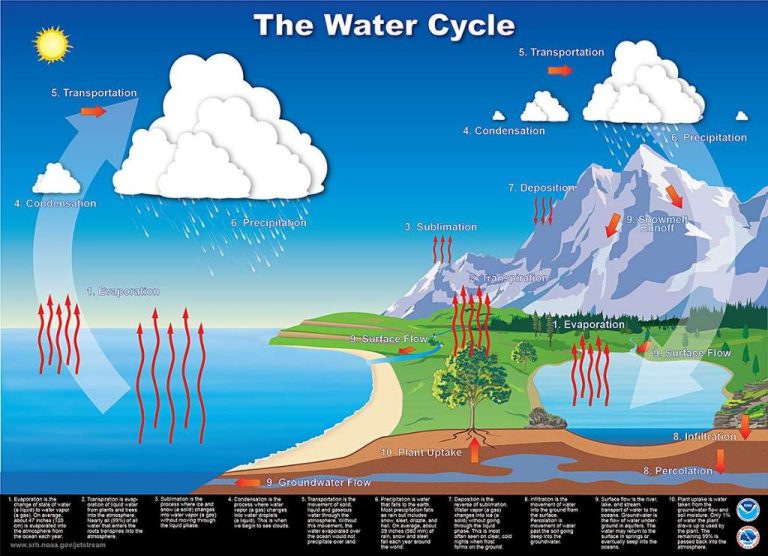
We explored many examples of energy converting from one form to another both in natural processes like photosynthesis, the water cycle, and food chains as well as in man-made systems like cars, power plants, and batteries. Efficiency is an important concept when examining energy conversions, and no process can ever convert 100% of the input energy into usable output energy.
Equations like E=mc^2 show the relationship between matter and energy, while the laws of thermodynamics govern energy conversions and heat transfers. Understanding how energy flows through systems enables scientists and engineers to harness it for useful work in technologies that have shaped human civilization.
While energy cannot be created or destroyed, it can change forms. With advanced knowledge of these transformations, we gain power over nature to improve lives. However, we must use this power responsibly by maximizing efficiency and developing sustainable energy solutions for the future.