Can Any Form Of Energy Change To Another?
Energy is the ability to do work or produce heat. There are many different forms of energy such as mechanical, thermal, chemical, electrical, nuclear, light, and sound energy. The law of conservation of energy states that energy can neither be created nor destroyed, it can only change from one form to another. This means the total energy in an isolated system always remains constant.
For example, when a ball drops, its potential energy changes to kinetic energy. The chemical energy stored in batteries can be converted to electrical energy to power devices. Coal stores chemical energy which is released as thermal energy when burned. This law allows energy to change between different types but the total amount of energy never changes.
Mechanical Energy
Mechanical energy is the energy possessed by an object due to its motion or position. There are two forms of mechanical energy: kinetic energy and potential energy.
Kinetic energy is the energy an object possesses due to its motion. The faster an object moves, the more kinetic energy it has. Some examples of kinetic energy include a moving car, flowing water, or the motion of electrons in a wire. Kinetic energy can be transferred between objects through collisions. For example, when a moving pool ball strikes a stationary one, it transfers some of its kinetic energy to start the stationary ball moving.
Potential energy is the stored energy an object possesses due to its position or shape. There are several types of potential energy: gravitational (dependent on height), elastic (dependent on deformation of an elastic object), and chemical (energy stored in the bonds between atoms). A rock sitting at the top of a hill or water held behind a dam are examples of gravitational potential energy. When released, this potential energy is converted into kinetic energy as the objects fall. Elastic potential energy can be seen in stretched springs or rubber bands. This potential energy transforms into kinetic energy if the elastic object is released.
Kinetic and potential energy can easily change between each other. For example, when you throw a ball into the air, you transfer energy from your body’s kinetic energy to the ball, giving it kinetic energy. As it rises, this kinetic energy transforms into gravitational potential energy. At the ball’s highest point, its potential energy is at maximum, and its kinetic energy is zero. As it falls, the potential energy converts back into kinetic energy.
Thermal Energy
Thermal energy refers to the internal energy present in substances due to the motion and interactions of their particles. It is directly related to the temperature of matter. The higher the temperature, the greater the thermal energy. Thermal energy is produced when work is done on a substance or when energy transformation takes place between substances. For example, rubbing your hands together produces thermal energy through friction. The faster the motion of particles in a substance, the more thermal energy it possesses.
Thermal energy is a form of kinetic energy and it can be transferred from one object to another in three main ways: conduction, convection, and radiation. Conduction is the transfer of heat between objects in direct contact with each other, like a spoon in a hot cup of coffee. Convection is the transfer of heat through the movement of fluids, like hot air rising or water boiling. Radiation is the transfer of heat in the form of infrared waves that travel through space, like the warmth from a fireplace radiating throughout a room.
Many other forms of energy can change into thermal energy. For example, mechanical energy from friction converts into thermal energy. Electrical energy flowing through wires converts into thermal energy. Chemical energy from burning fuel converts into thermal energy. Nuclear energy from nuclear reactions converts into thermal energy. Even light energy from the sun can be absorbed and converted into thermal energy when it strikes an object. So thermal energy acts as a bridge allowing the transformation of other energy types into heat.
Chemical Energy
Chemical energy is the energy stored in the bonds between atoms and molecules. It is the energy that holds atoms together in chemical compounds. Chemical energy can be stored in chemicals and released for uses by chemical reactions. Common examples of stored chemical energy are in batteries, food, fuel, etc.
There are two main types of chemical reactions that can release the stored chemical energy – exothermic and endothermic reactions. In exothermic reactions, energy is released, usually in the form of heat, light, or sound. Examples are burning of wood, digestion of food, etc. In endothermic reactions, energy is absorbed and stored in the chemical bonds. Examples are photosynthesis in plants, charging of batteries, etc.
The chemical energy stored in molecules can be converted into other forms of energy through chemical reactions. The breakdown of food in cellular respiration converts the stored chemical energy into thermal, mechanical and electrical energy that cells use for metabolism. In batteries, chemical reactions convert stored chemical energy into electrical energy. In nuclear reactors, nuclear energy is released when specific isotopes undergo nuclear fission or fusion reactions. Combustion of fuels like wood, coal, petrol, etc. release heat and light energy through exothermic chemical reactions.
Therefore, chemical energy can interconvert into thermal, electrical, mechanical, light, sound and nuclear energy through chemical reactions. The law of conservation of energy applies to these energy transformations. While chemical energy can change into other forms of energy, some amount of energy is always lost to the surroundings as heat in the process.
Electrical Energy
Electrical energy refers to the movement of electrons, which creates an electric current. This current can be generated through various energy conversions. For example, mechanical energy from moving water or wind can spin turbines connected to generators to produce electricity. The motion is converted into electrical current. Similarly, solar panels convert light energy from the sun into electrical current through the photovoltaic effect. Chemical reactions like combustion or batteries can also generate electricity through electrochemical processes.
The voltage, or electromotive force, measures the pressure or potential for electrons to flow as electrical current. Higher voltage corresponds to more potential energy built up to push the electrons. When there is a path to flow, the electrons release their energy to power electrical devices and equipment. The current is the rate of electrical charge flow. So voltage provides the driving force, while current is the measurable outcome.
Many other forms of energy like mechanical, thermal, nuclear, light, and chemical can undergo conversions to produce electrical energy through generators, solar cells, batteries, etc. Electricity is an extremely useful form of energy that allows powering modern society.
Nuclear Energy
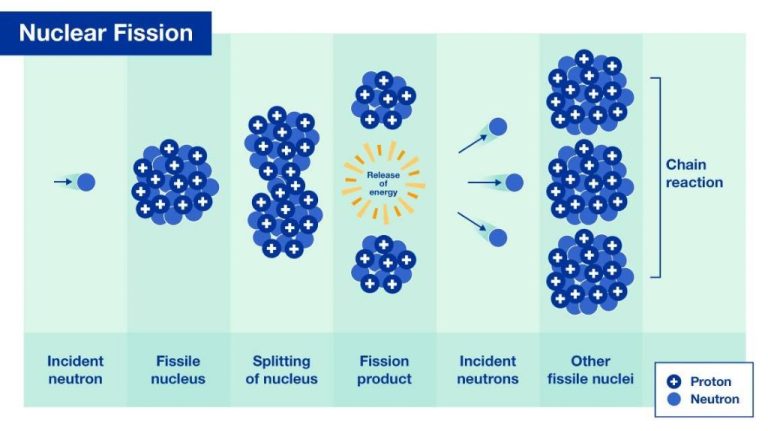
Nuclear energy comes from the splitting (fission) or merging (fusion) of atomic nuclei. In nuclear fission, the nucleus of a heavy atom like uranium or plutonium is split into two smaller nuclei, releasing a large amount of energy in the process. This energy can be used to generate electricity. In nuclear power plants, the fission of uranium atoms in the reactor core produces heat that is used to boil water into steam. The steam spins a turbine connected to a generator to produce electricity.
In nuclear fusion, atomic nuclei are fused together to form heavier nuclei, also releasing energy. Fusion occurs naturally in stars like our sun. Scientists are researching ways to control fusion reactions to harness this energy source on Earth. Fusion power offers the promise of nearly limitless energy with much less radioactive waste than fission. However, fusion technology is still in early experimental stages.
Both fission and fusion release energy according to Einstein’s equation, E=mc2. Even a small amount of mass converted during these reactions releases an enormous amount of energy, millions of times more than from chemical reactions like burning fossil fuels. This makes nuclear power extremely efficient, able to generate large amounts of electricity from a very small amount of fuel.
Light Energy
Light energy is a form of radiant energy that can be detected by the human eye. It consists of electromagnetic particles called photons, which exhibit properties of both waves and particles. Light energy travels in a straight line until it interacts with matter.
There are a few key ways that light energy interacts with matter:
-
Reflection – Light bounces off smooth, shiny surfaces like mirrors. The angle of reflection equals the angle of incidence.
-
Refraction – Light bends when passing from one medium to another at an angle. For example, a straw in water appears bent due to the change in light’s velocity.
-
Absorption – Light energy can be absorbed by matter and converted to other forms of energy. Dark, rough surfaces tend to absorb more light.
-
Transmission – Some materials like glass allow most visible light to pass through unchanged.
-
Scattering – Small particles in the air scatter light in different directions, enabling us to see beams of light.
-
Diffraction – Light bends and spreads out when passing through small openings or around sharp edges.
Understanding how light interacts with matter has enabled many useful technologies like fiber optics, solar panels, cameras and more.
Sound Energy
Sound energy is produced when an object vibrates. For example, when someone speaks their vocal cords vibrate to create sound waves that transmit energy through the air. Musical instruments like guitars also work by vibrating strings or membranes to create sound energy. The vibrations cause particles in the air to move, transmitting energy through the medium of air. Without the vibration, there would be no sound energy. The amount of energy in a sound wave relates to its amplitude (loudness) and frequency (pitch).
Sound energy can change forms as it travels and interacts with objects. For example, when sound waves hit a wall, some of the energy may be absorbed by the wall, causing it to vibrate slightly and transforming the sound energy into mechanical energy. The rest of the sound wave reflects off the wall. Electrical energy can also be converted into sound energy, such as when a loudspeaker transforms electrical signals into audible sound. Sound energy dissipates and becomes more dispersed as it travels through the air until it becomes so scattered it is no longer detectable. The law of conservation of energy applies – the sound energy changes forms but the total amount of energy remains constant.
Limitations
While energy can change from one form to another, these conversions are never 100% efficient. There are always some inefficiencies and energy losses in any energy conversion process. Some key limitations include:
Friction and Heat Losses: When converting mechanical energy to electrical energy, friction in moving parts causes energy to be lost in the form of heat. This heat is dissipated and cannot be fully converted.
Resistance Losses: When converting electrical energy to another form, the resistance in wires causes some energy to be lost as heat. This reduces efficiency.
Sound and Light Energy: When converting other forms of energy to sound or light energy, not all the initial energy can be converted. Sound and light dissipate over distance.
Nuclear and Chemical Processes: Converting nuclear energy to electrical energy or chemical energy to mechanical energy involves complex processes that cannot capture all the initial energy.
Due to these inefficiencies, perpetual motion machines that can convert energy with 100% efficiency are not possible. However, work continues to maximize efficiency and minimize energy losses in conversion processes.
Conclusion
The changes and transferences between different forms of energy are made possible through various conversion processes. While energy can change from one form to another, it is never created or destroyed, only converted. Through mechanical, thermal, chemical, electrical, nuclear, light, and sound energy conversions, the total sum of energy in a closed system remains fixed.
Some key learnings are that mechanical energy like motion or position can change into thermal energy like heat. Chemical energy stored in molecules and compounds can convert into thermal, mechanical, electrical, and light energy. Nuclear energy from radioactive materials can become thermal and electrical energy. Light energy from sources like the sun can transform into thermal, chemical, and electrical energy. However, there are limitations to energy conversions – some transformations are easier than others, and not all forms of energy can directly change into another.
Overall, the different forms of energy are intimately connected through conversion processes. Energy flows between mechanical, thermal, chemical, electrical, nuclear, light, and sound based on the laws of thermodynamics. While energy conversions have limits and inefficiencies, the ability of energy to change form makes nearly all the processes and technologies of modern civilization possible.